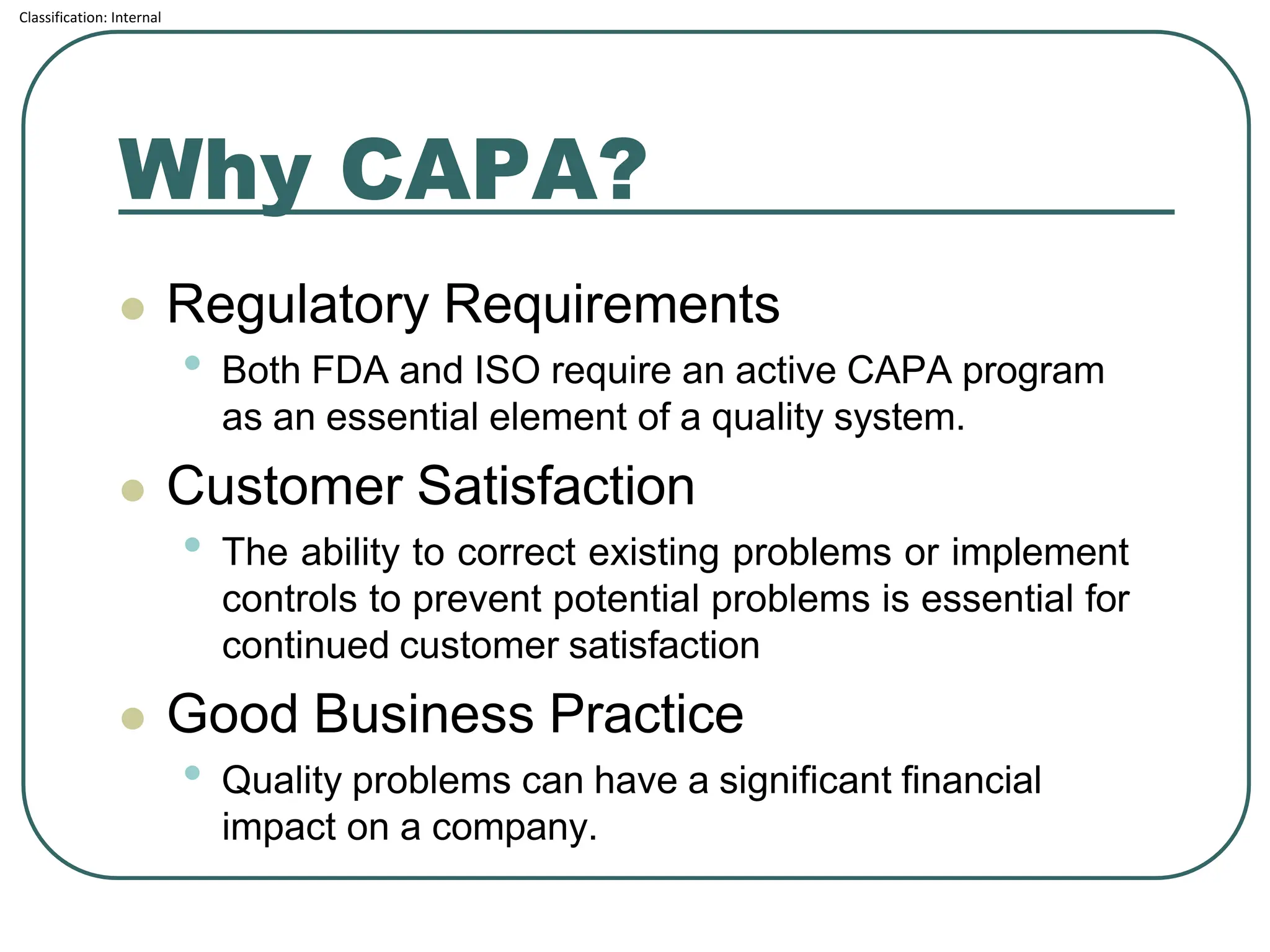
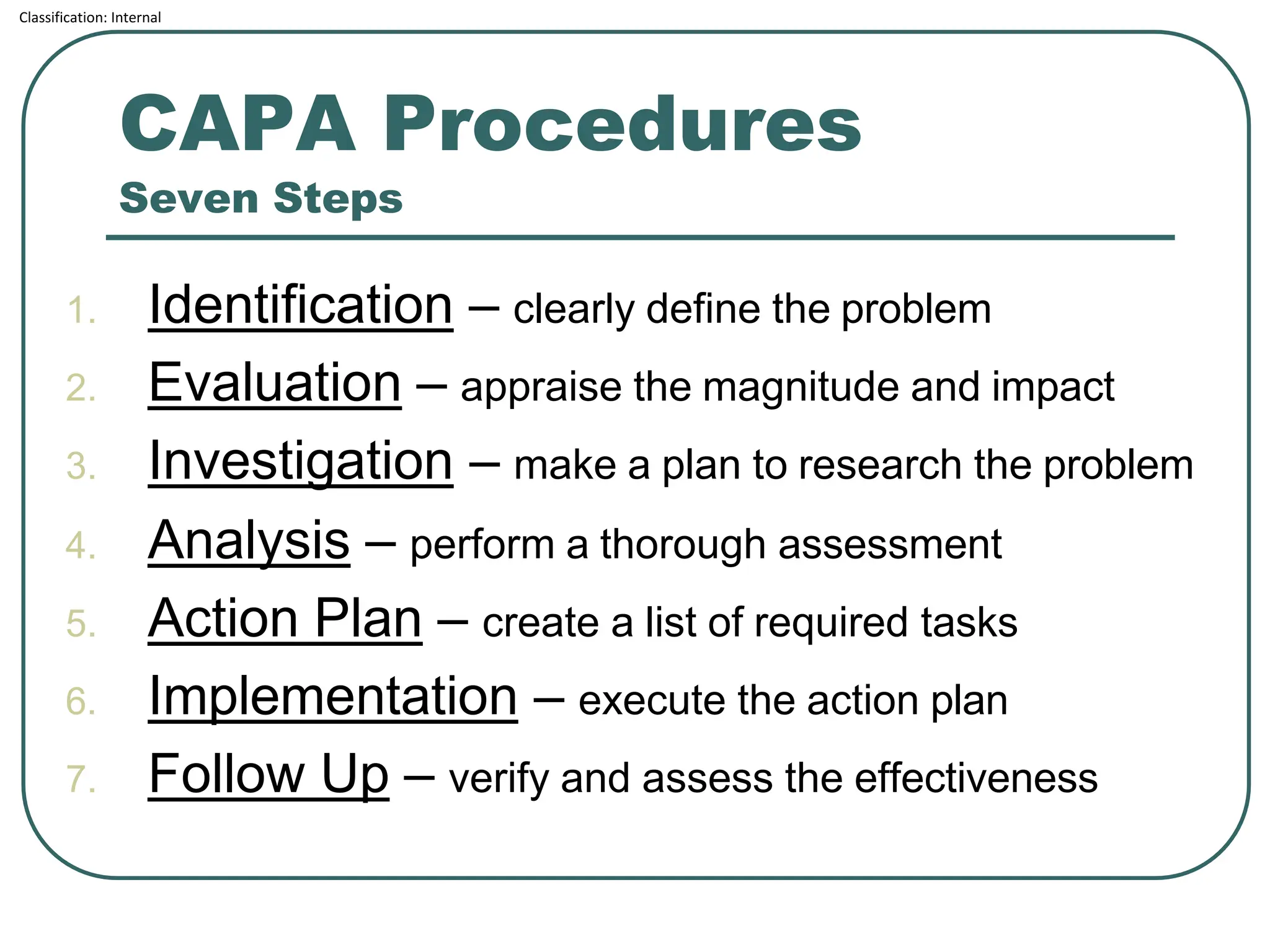
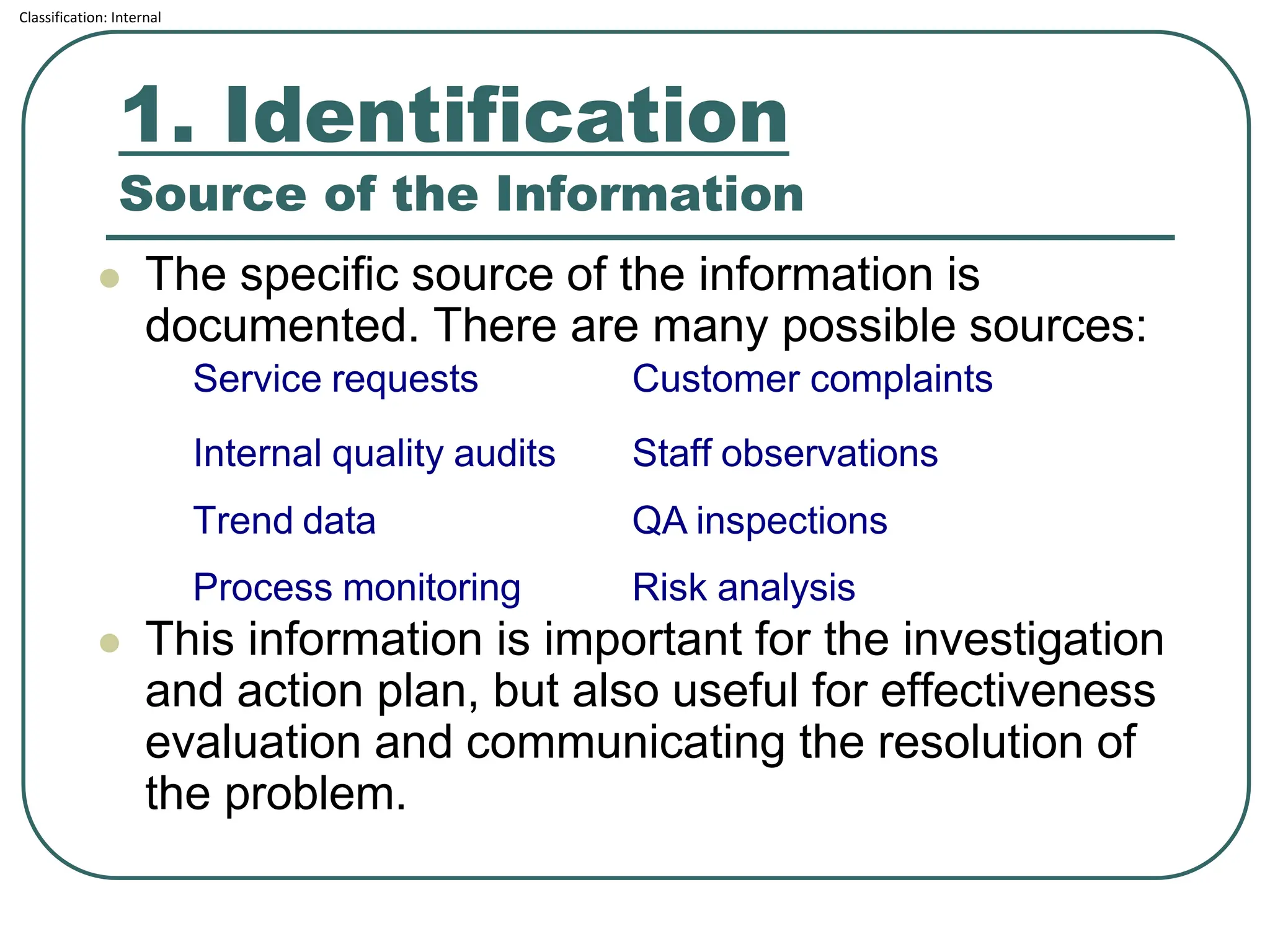
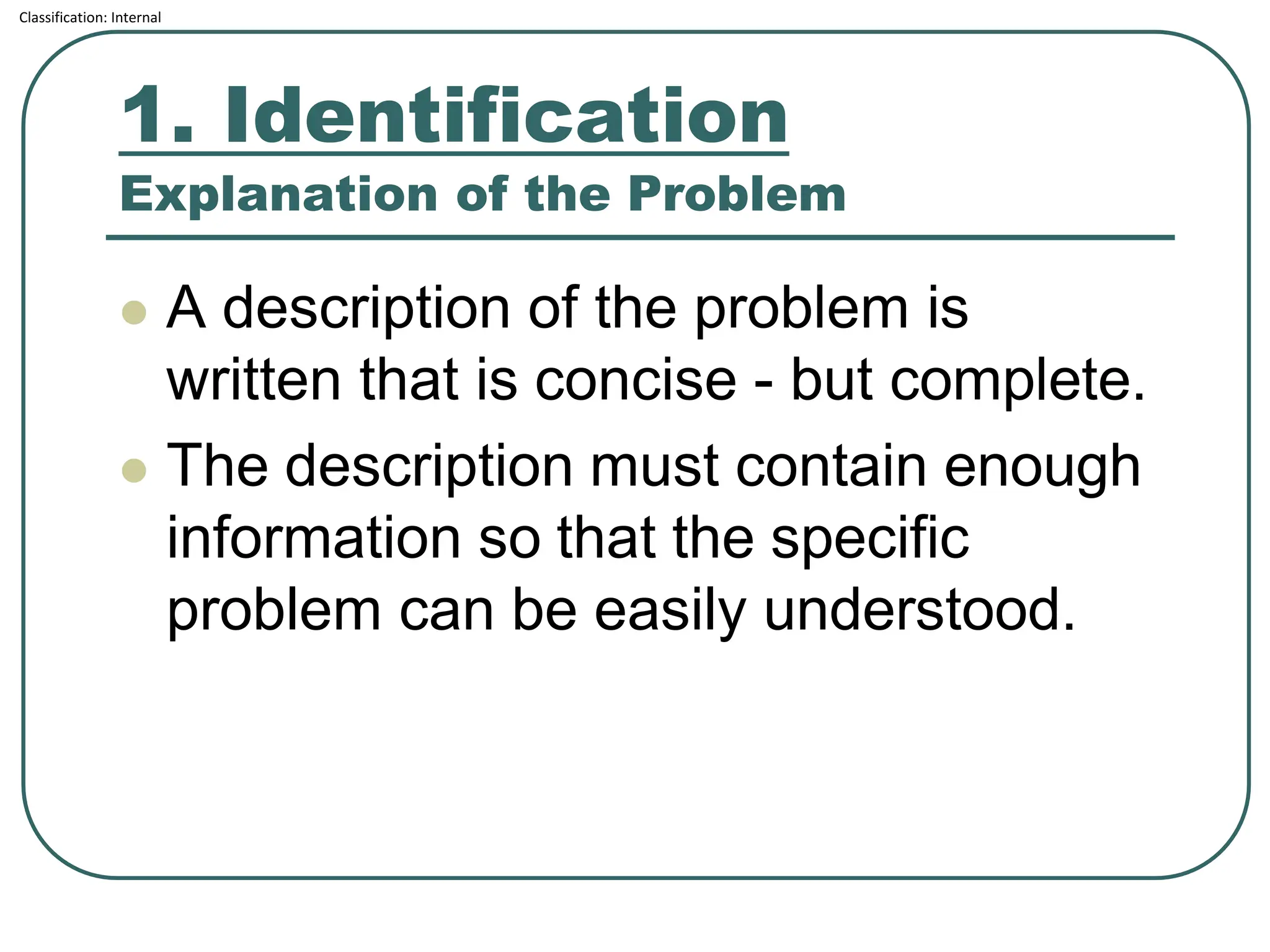
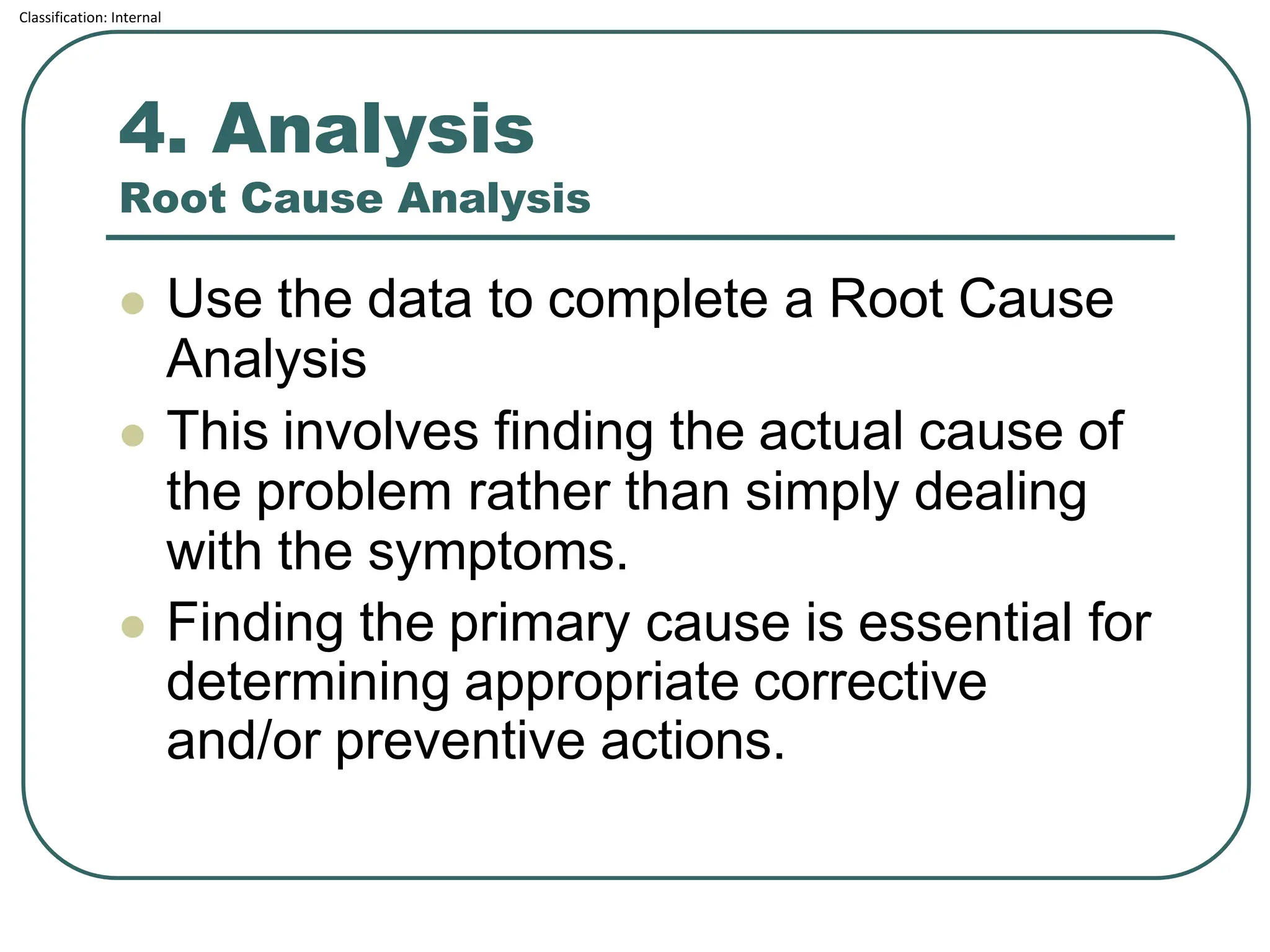
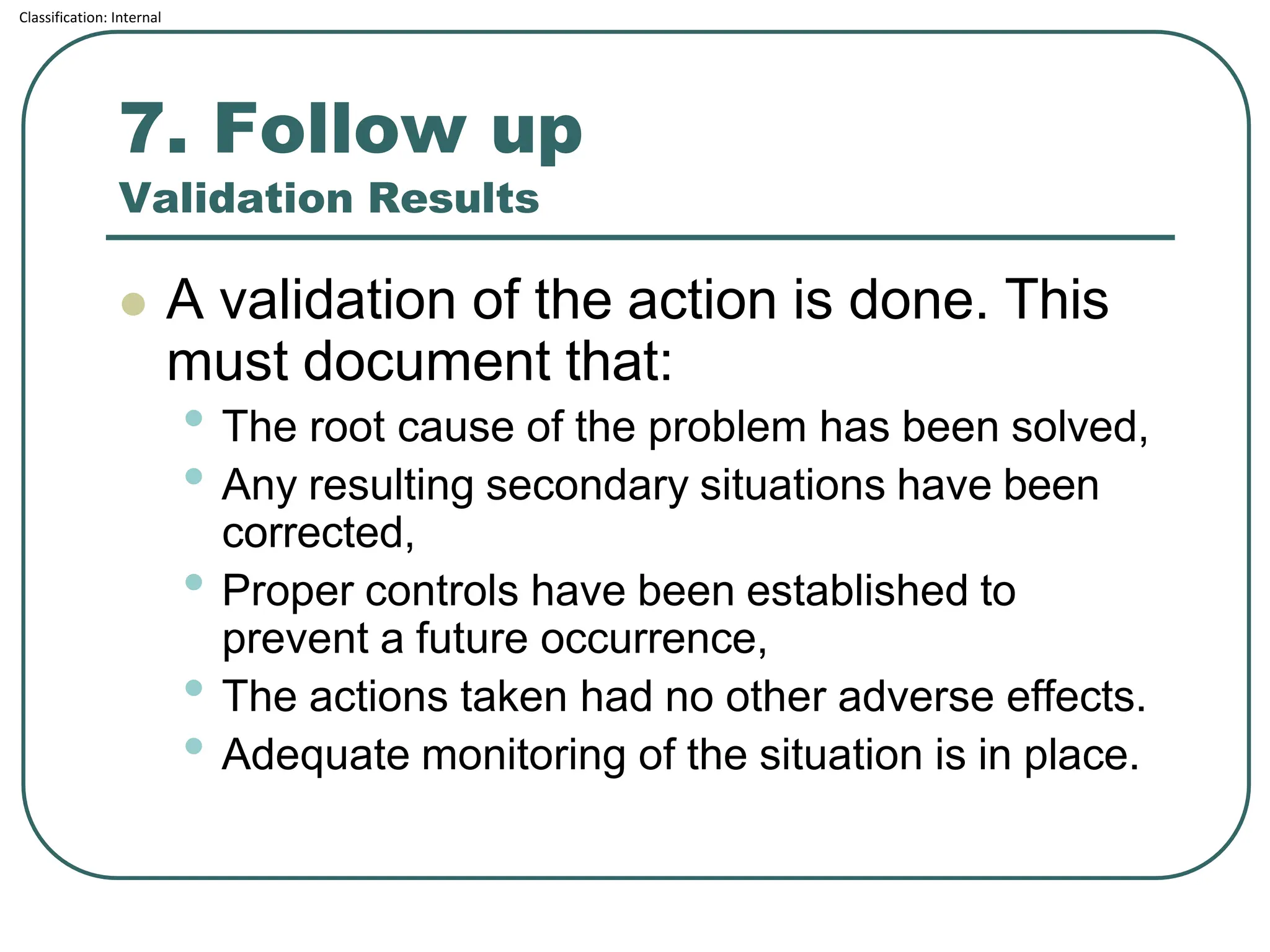
The document outlines the corrective and preventive actions (CAPA) classification, detailing the processes for addressing existing product issues and preventing potential problems, which are crucial for regulatory compliance, customer satisfaction, and good business practices. It details a seven-step CAPA process that includes identification, evaluation, investigation, analysis, action planning, implementation, and follow-up, emphasizing the importance of thorough documentation at each step. Examples of effective CAPA strategies include process redesign, training improvements, and error-proofing techniques.