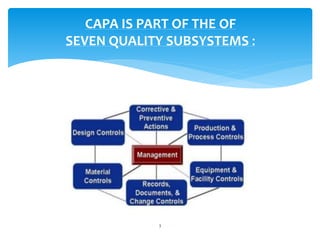
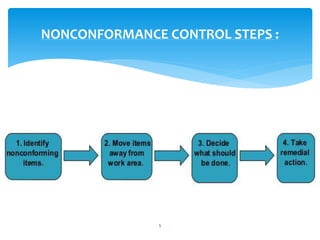
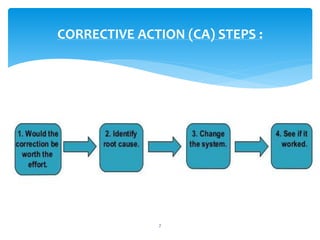
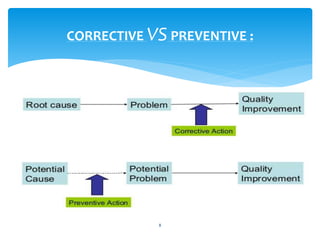
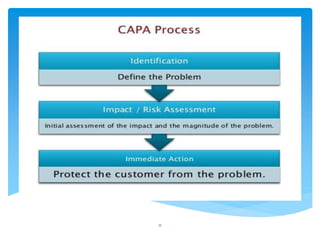
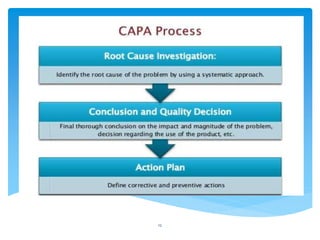
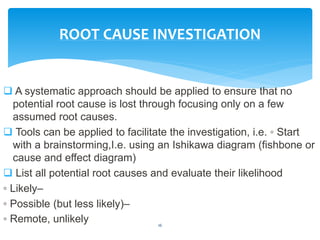
This document provides an overview of corrective and preventive actions (CAPA) as part of a quality management system. It defines CAPA and explains that it aims to eliminate causes of nonconformities and prevent their recurrence. The document outlines the CAPA process, which involves nonconformance identification, root cause analysis, corrective action planning, implementation, and follow-up to verify effectiveness. It emphasizes that CAPA is important for continuous quality improvement.