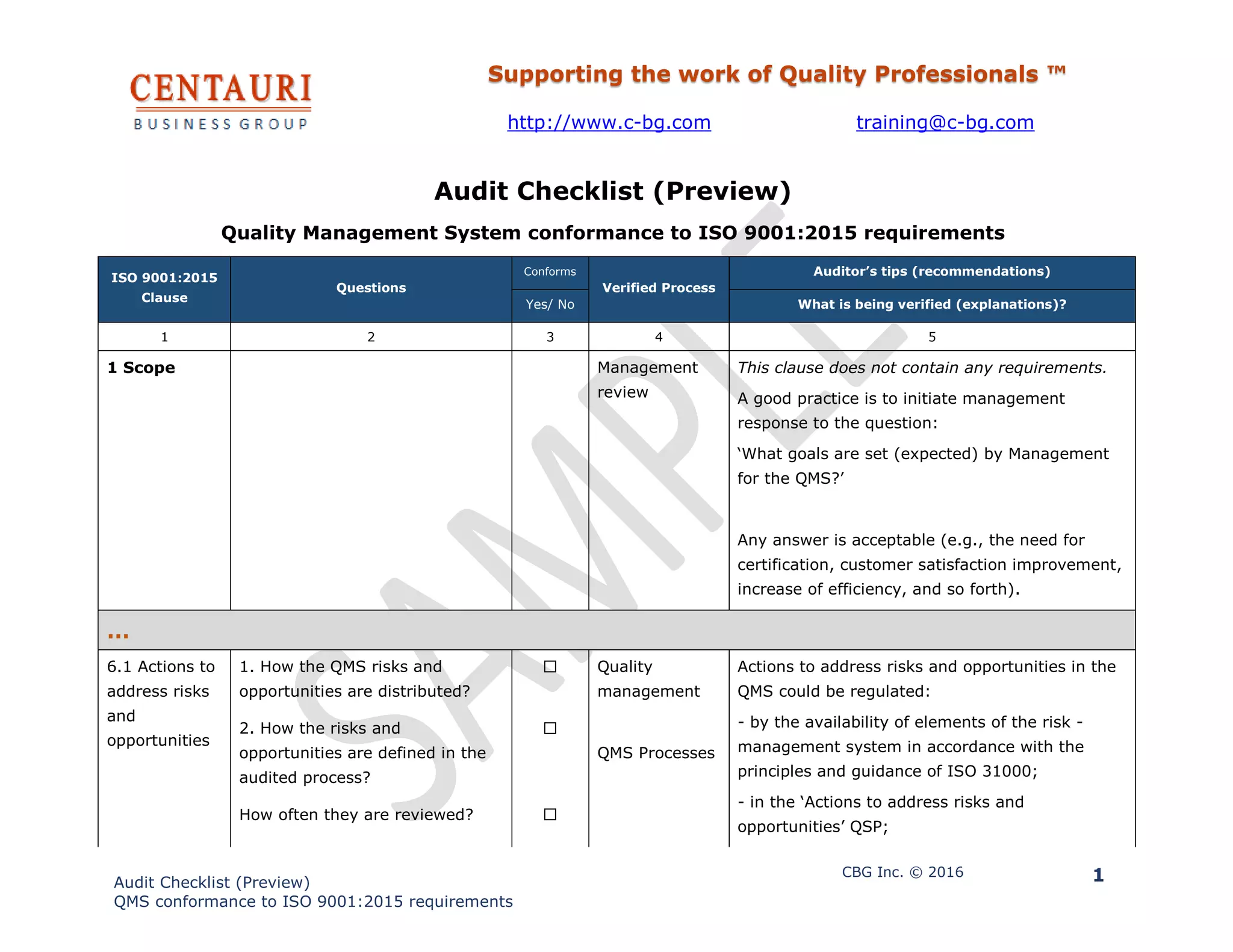
The document is an audit checklist designed to ensure compliance with ISO 9001:2015 standards for Quality Management Systems. It includes questions and recommendations for verifying whether various processes conform to specified clauses, covering topics like risk assessment, personnel competence, and process output preservation. The checklist serves as a tool for quality professionals to systematically evaluate and improve their organization's quality management practices.