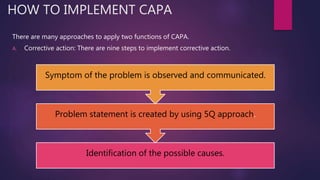
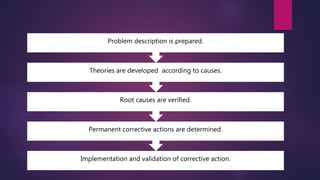
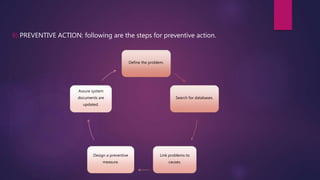
This document discusses corrective and preventive action (CAPA) systems. It defines key terms like nonconformance and defines CAPA's goals of eliminating causes of non-conformities. CAPA has two functions - corrective actions to address root causes of problems, and preventive actions to prevent reoccurrence. The document outlines objectives of an effective CAPA system and provides steps to implement corrective and preventive actions, including defining problems, identifying causes, designing measures, and ensuring documentation is updated. It stresses the importance of planning, communication, and documentation for successful CAPA execution.