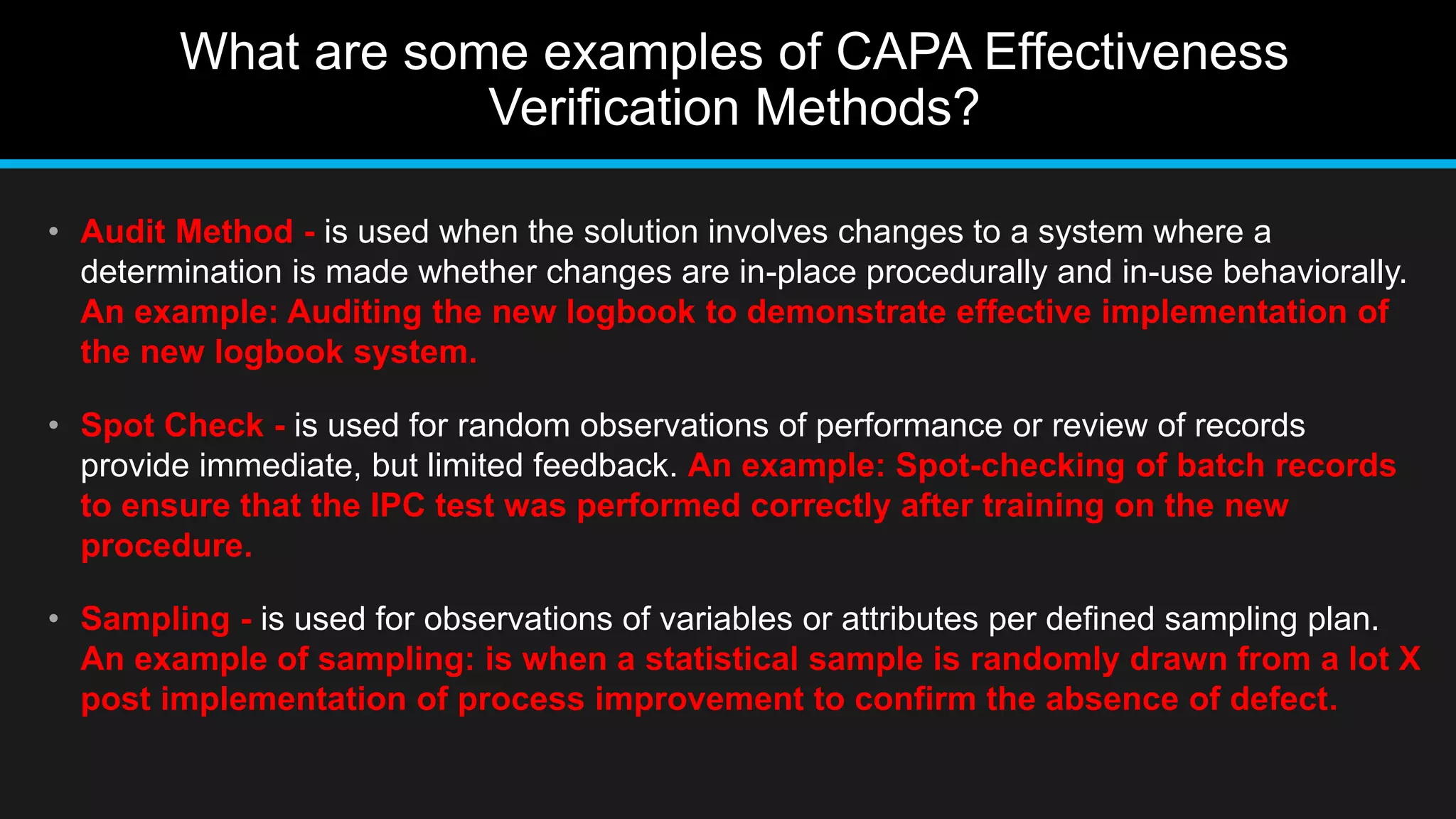
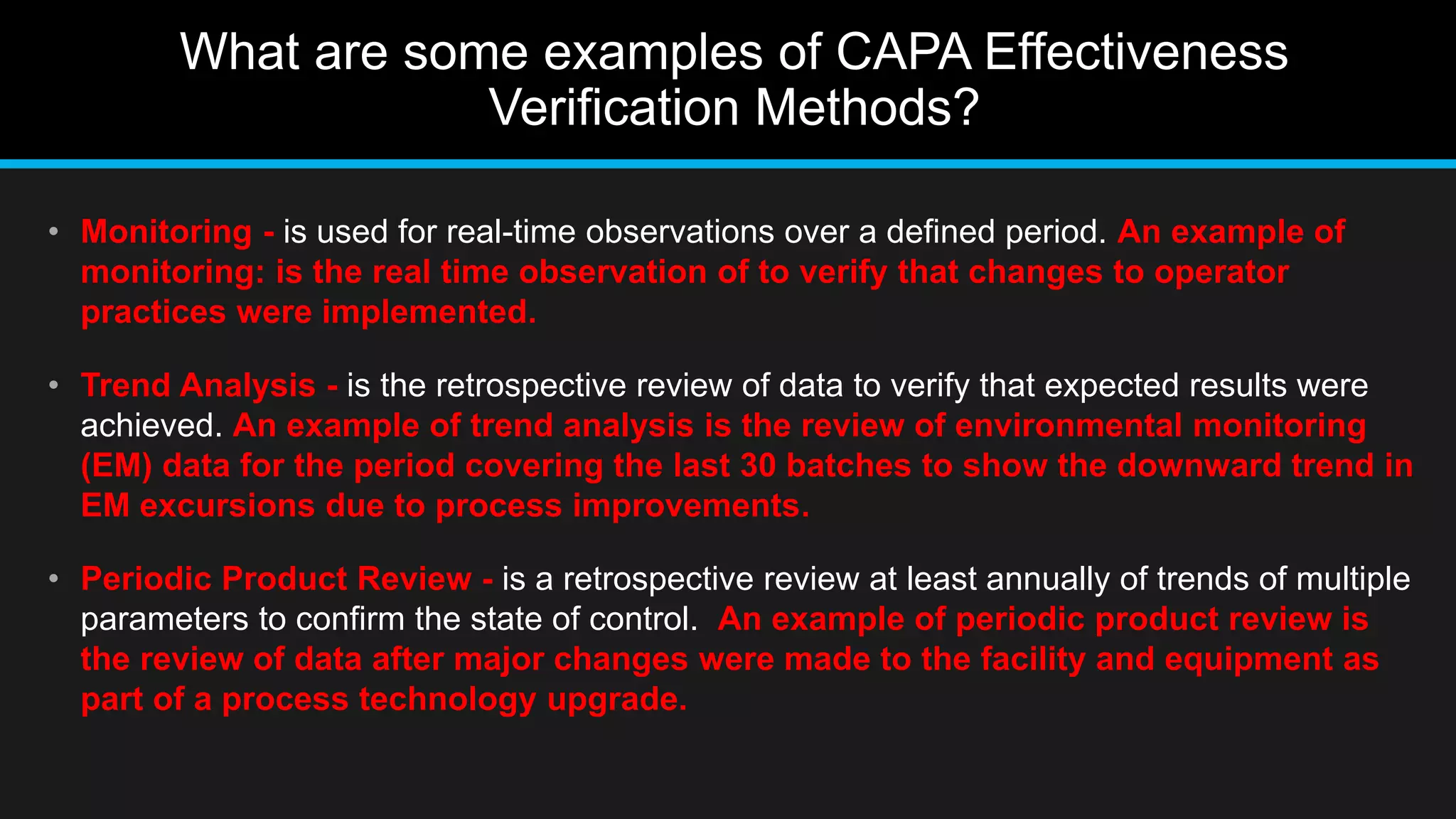
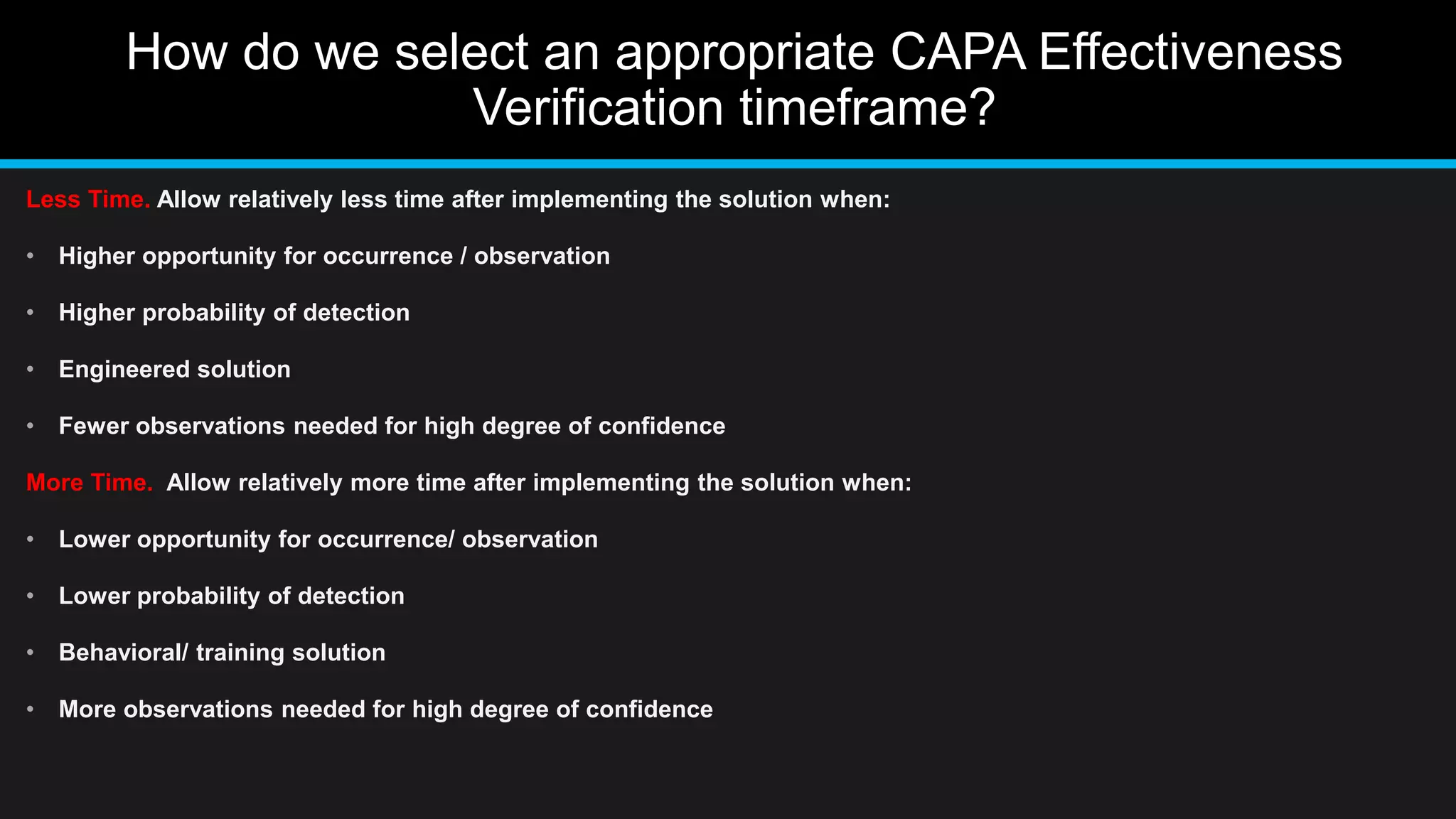
This document discusses 6 methods for verifying the effectiveness of corrective and preventive actions (CAPA). It identifies the difficulty in determining an appropriate CAPA effectiveness verification method when the problem is not well defined or the root cause is not determined. It then provides examples of 6 CAPA effectiveness verification methods: audit method, spot check, sampling, monitoring, trend analysis, and periodic product review. Finally, it discusses how to select an appropriate CAPA effectiveness verification timeframe, allowing less time for solutions with higher opportunity for observation and detection, and more time for solutions with lower opportunity for observation and detection.