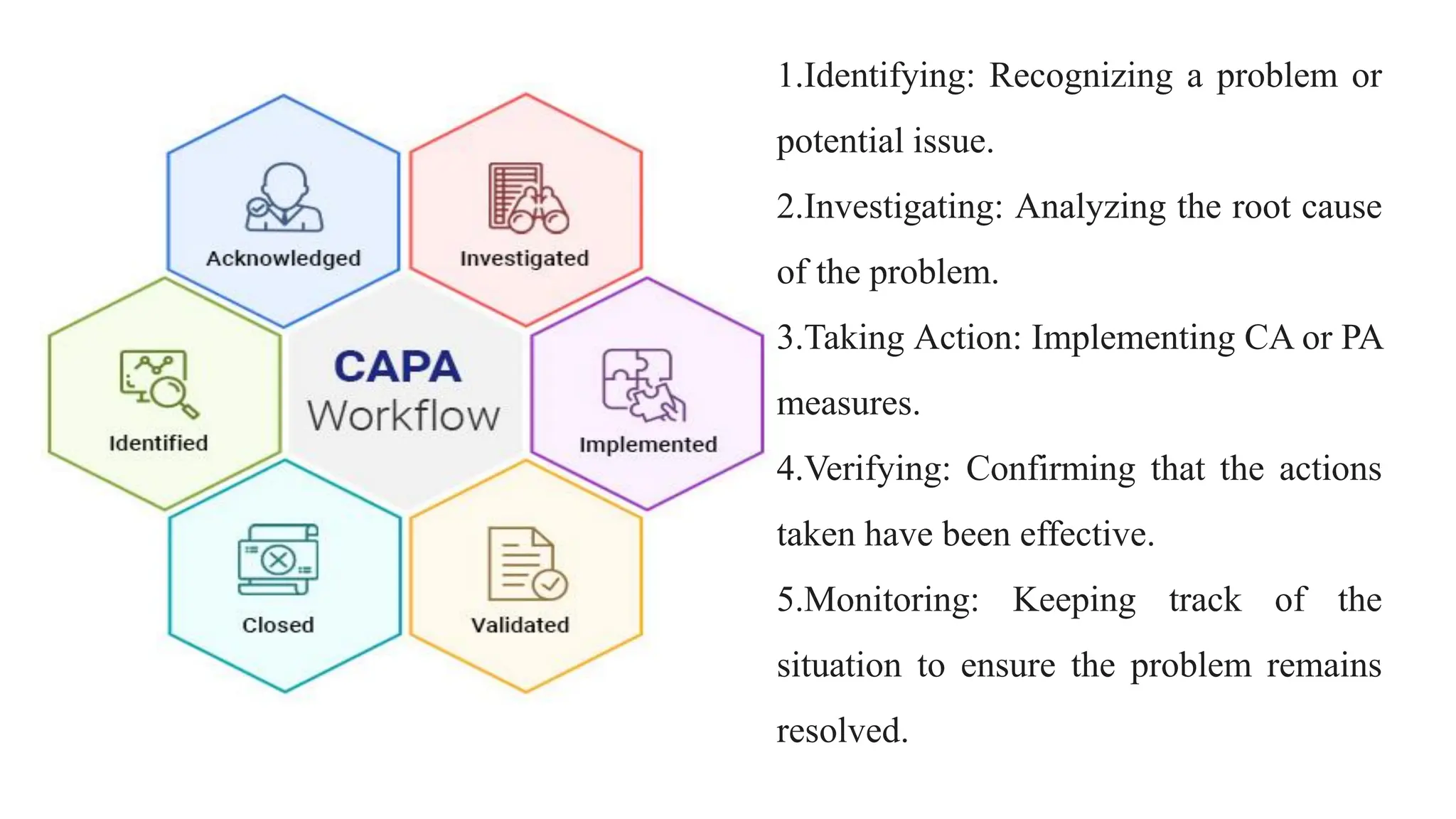
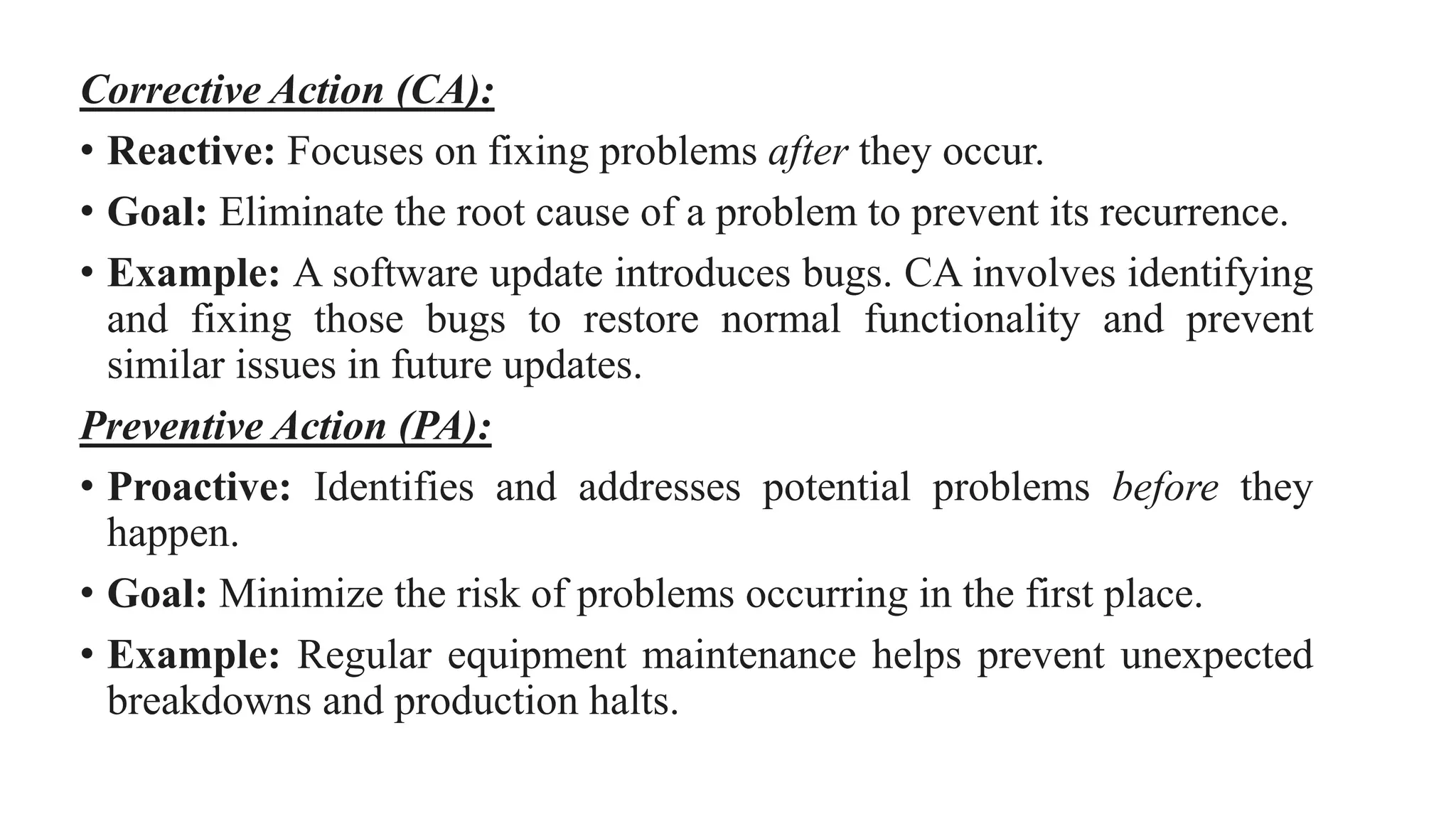
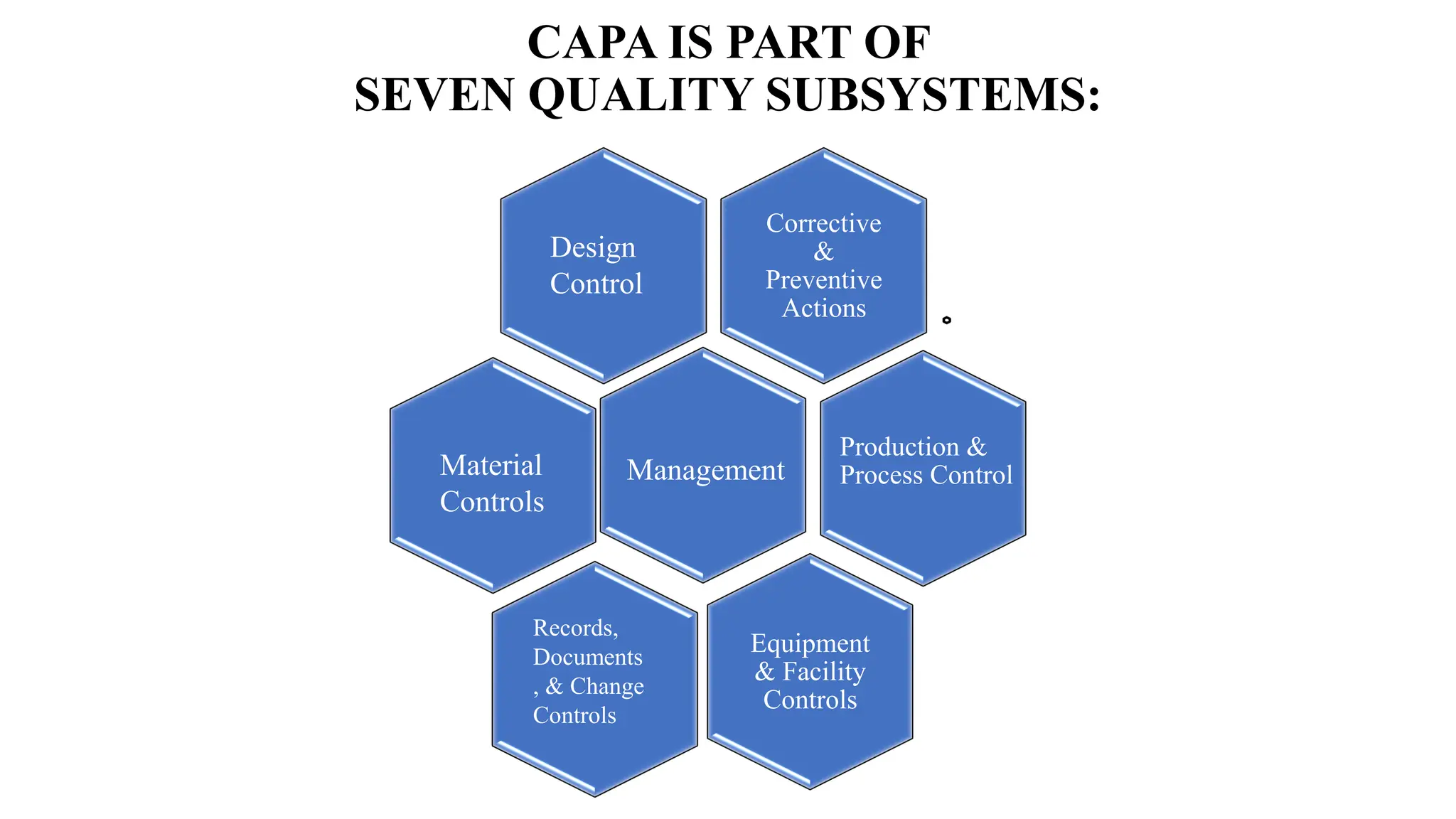
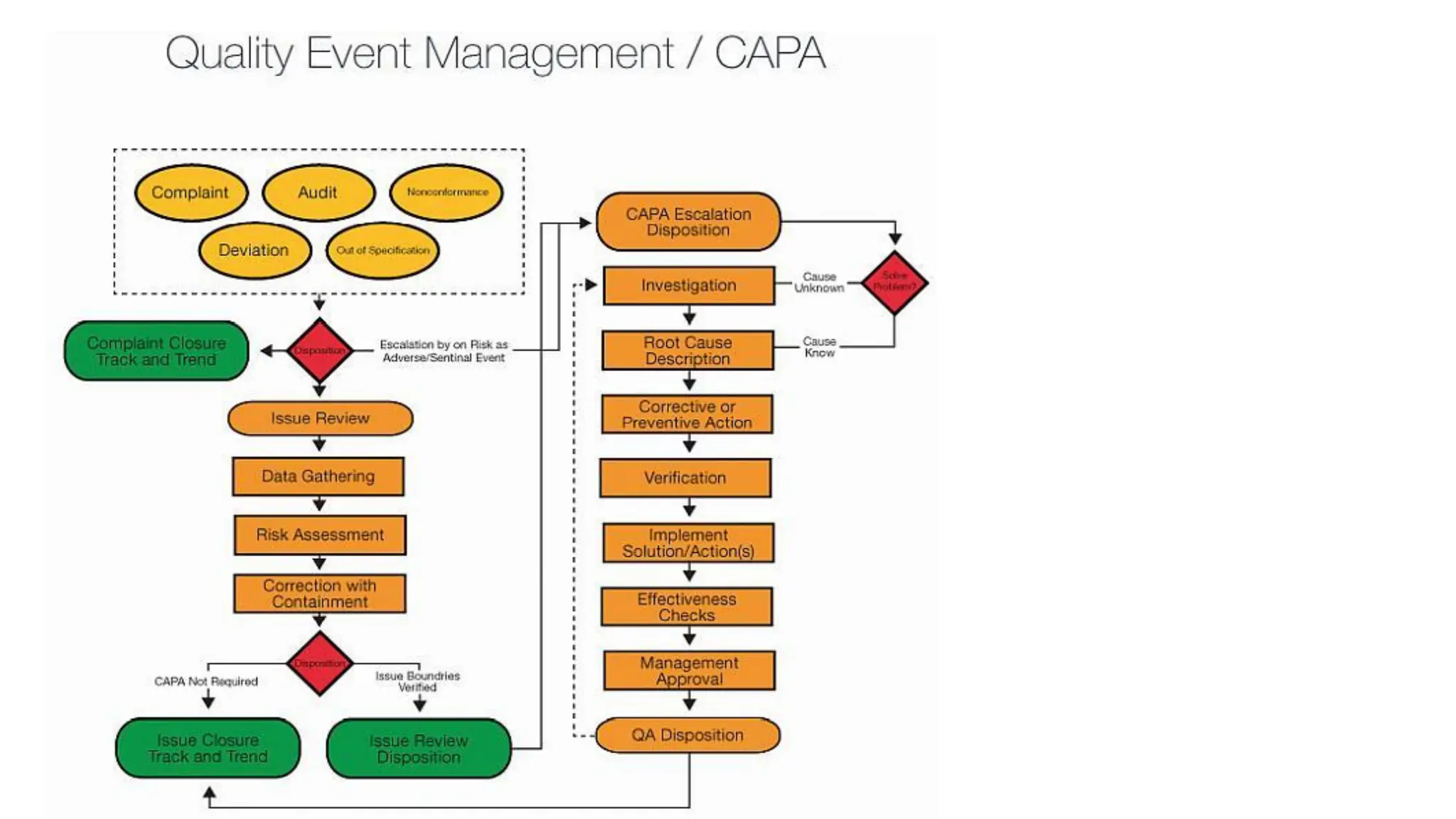
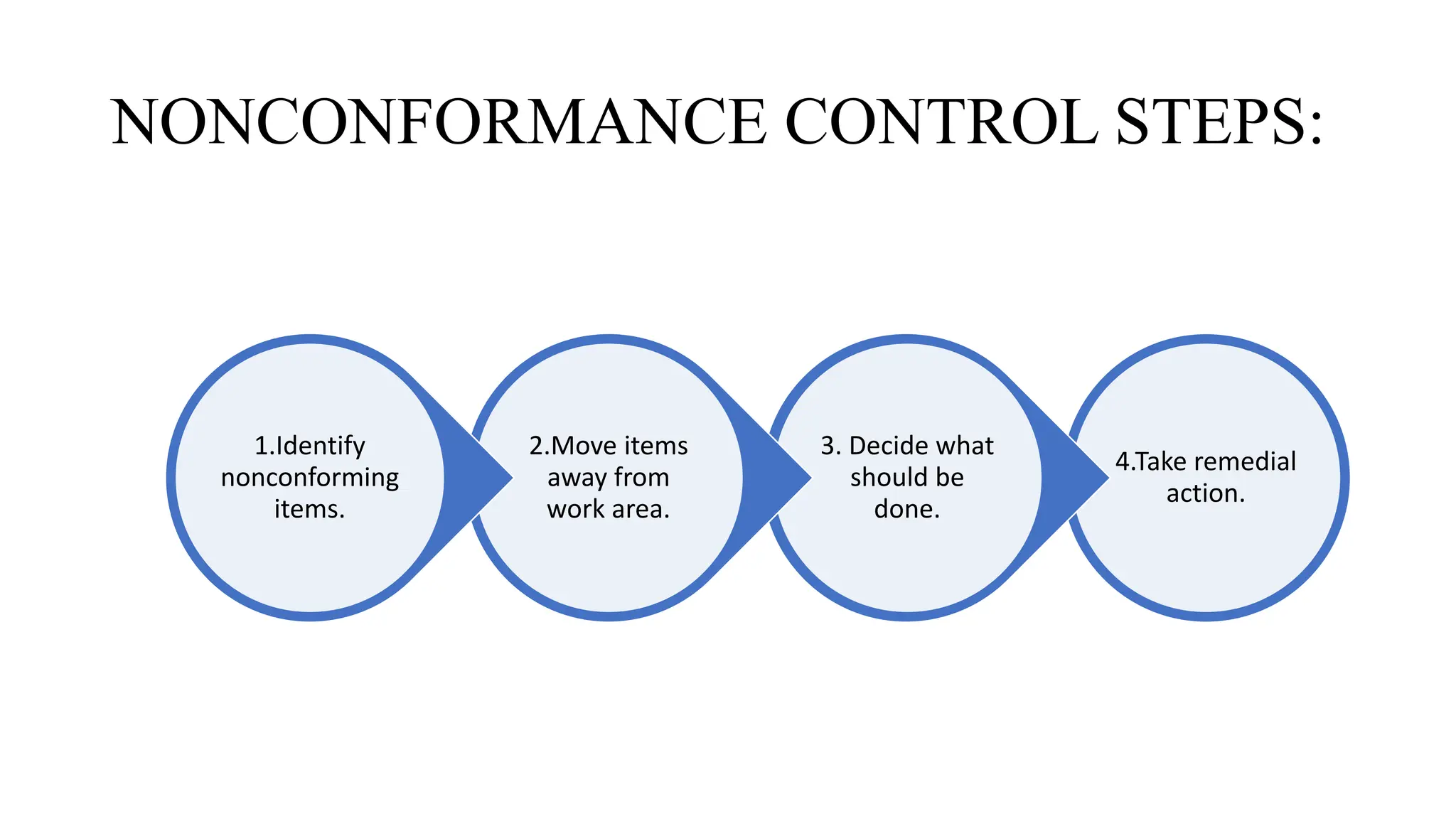
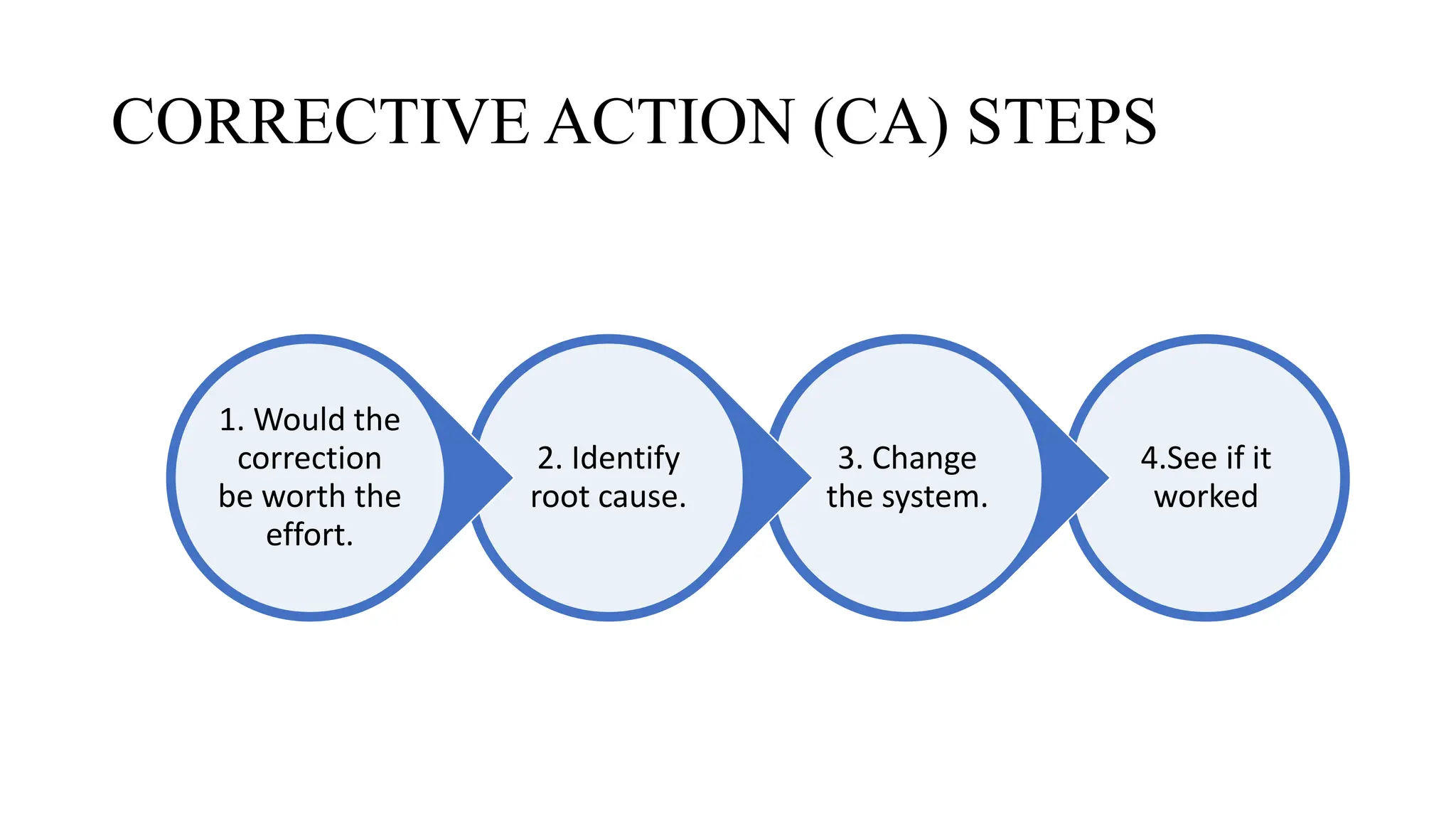
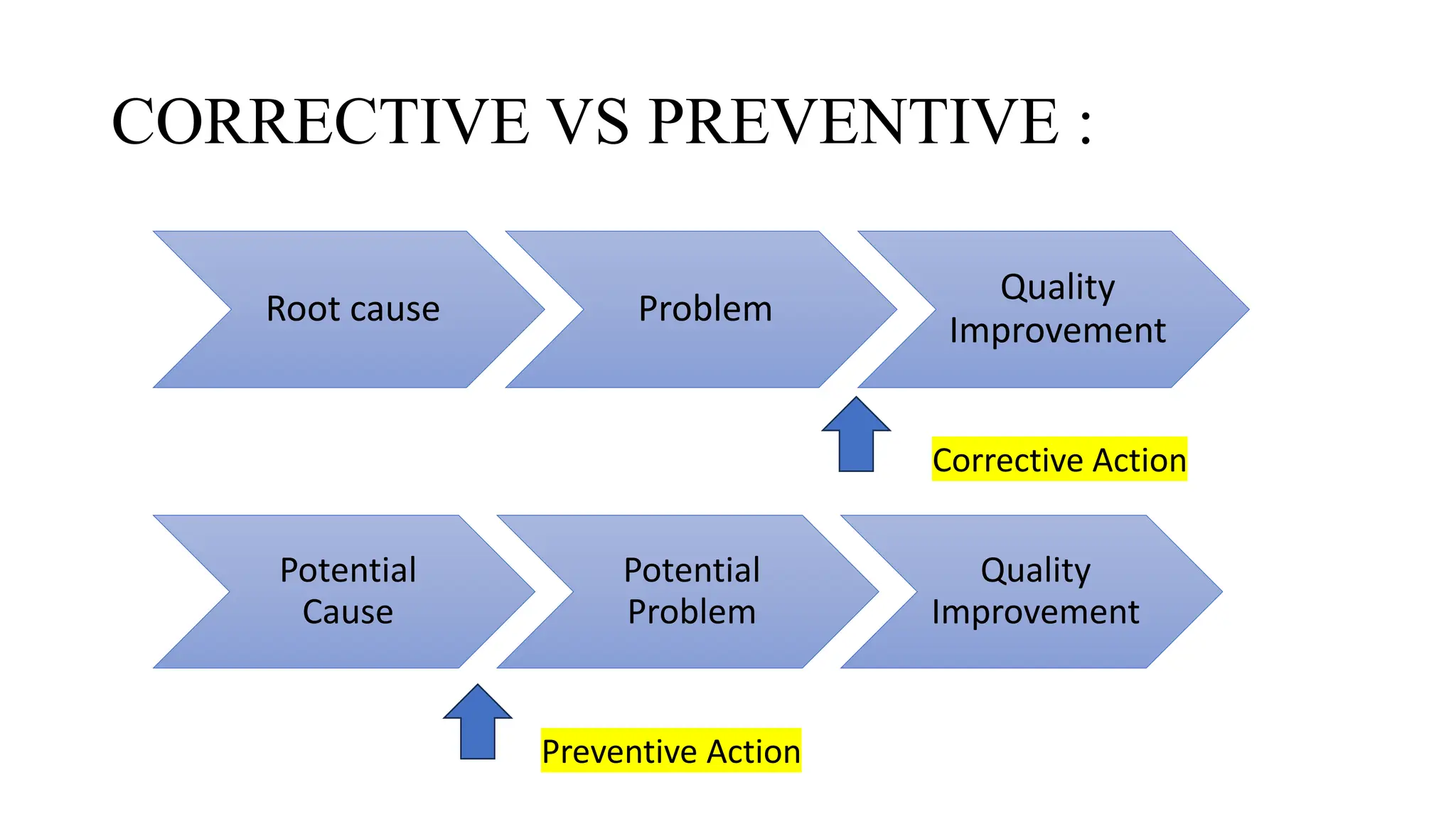
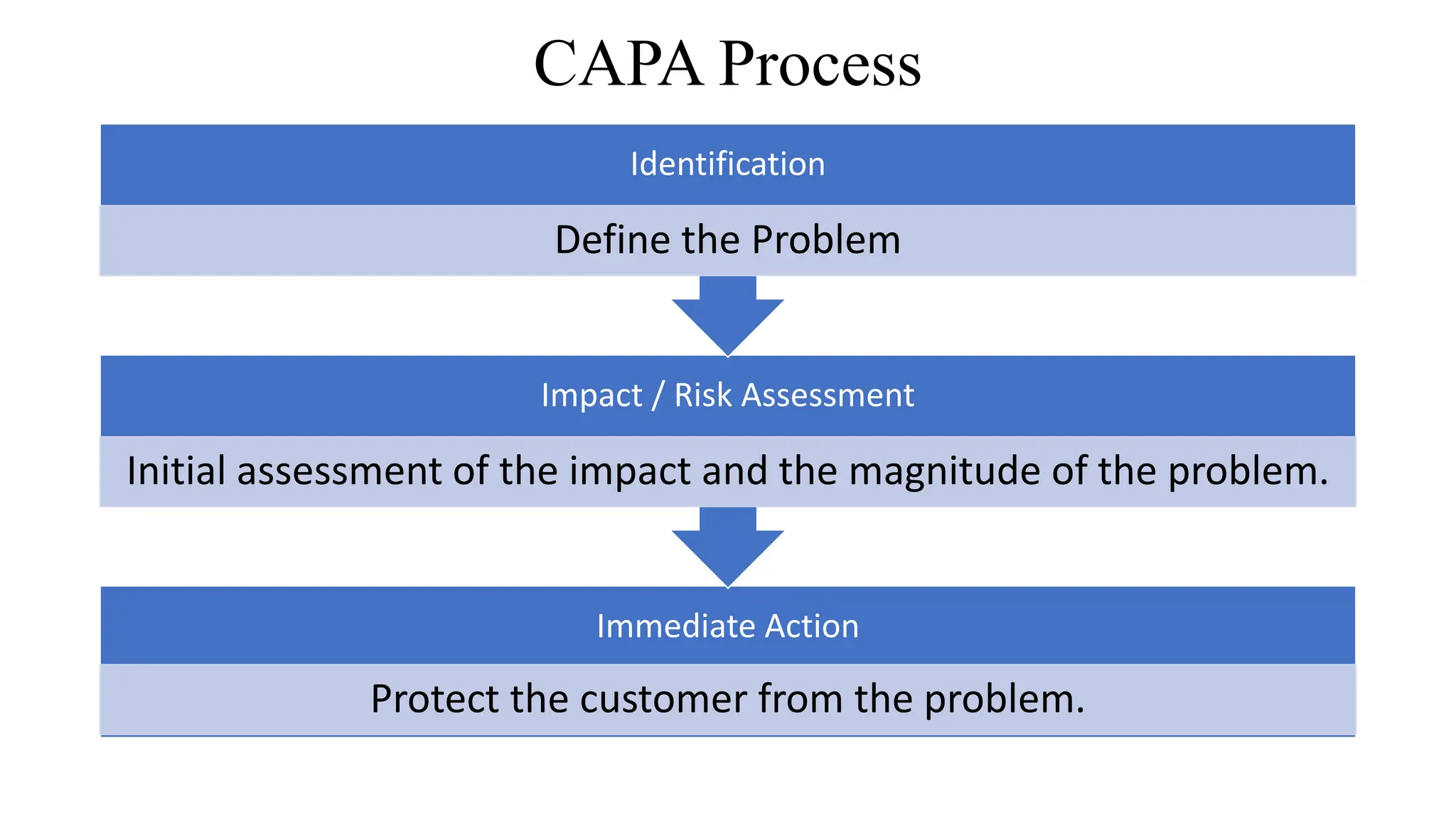
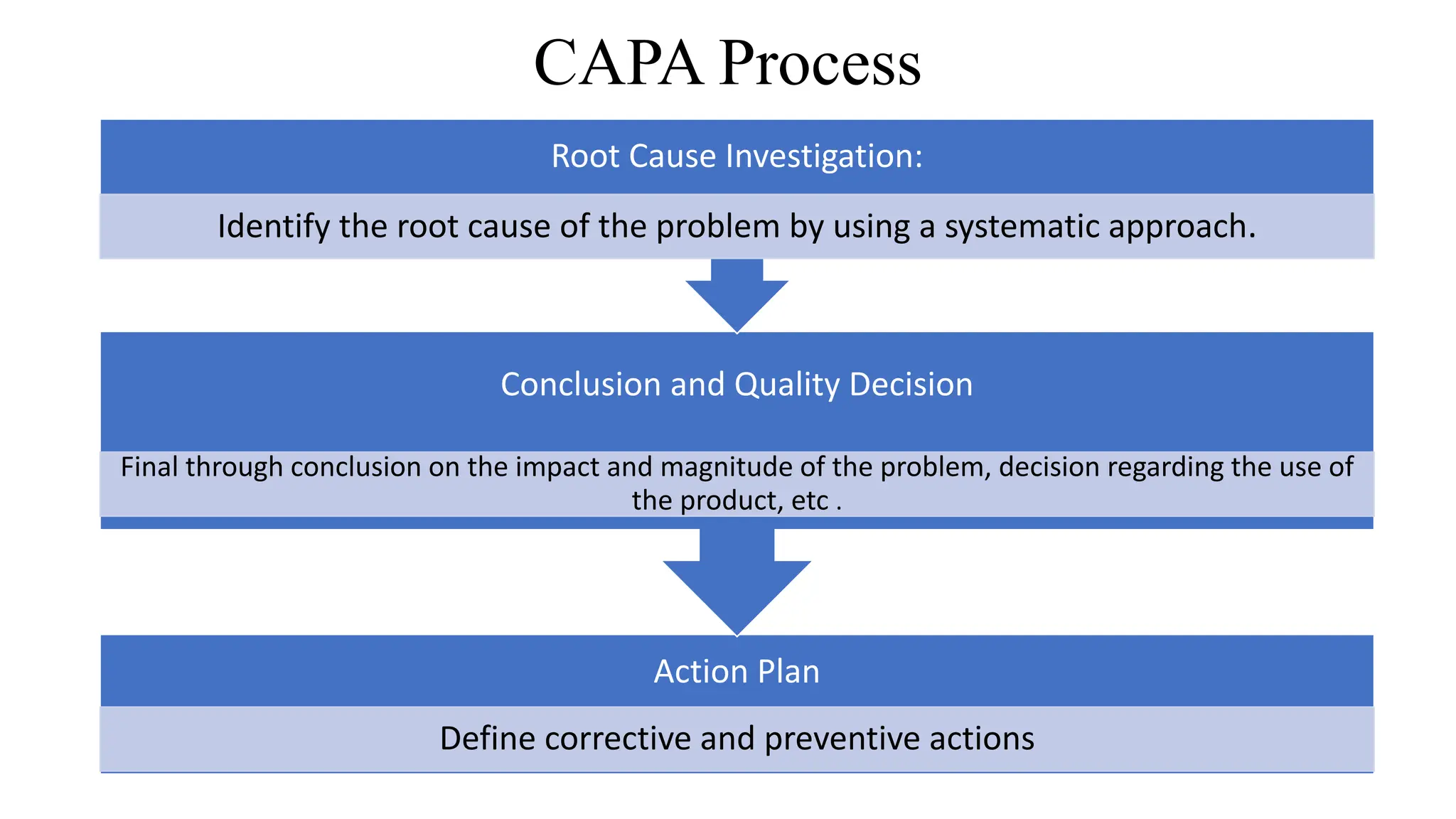
This document discusses corrective and preventive action (CAPA) processes. It defines CAPA and outlines the typical steps, including identifying issues, investigating root causes, taking action, verifying effectiveness and monitoring. Corrective action addresses existing problems, while preventive action aims to prevent potential future problems. The document provides examples and definitions of key CAPA terms and principles. It emphasizes that CAPA is an important part of a quality management system and helps ensure continuous quality improvement.