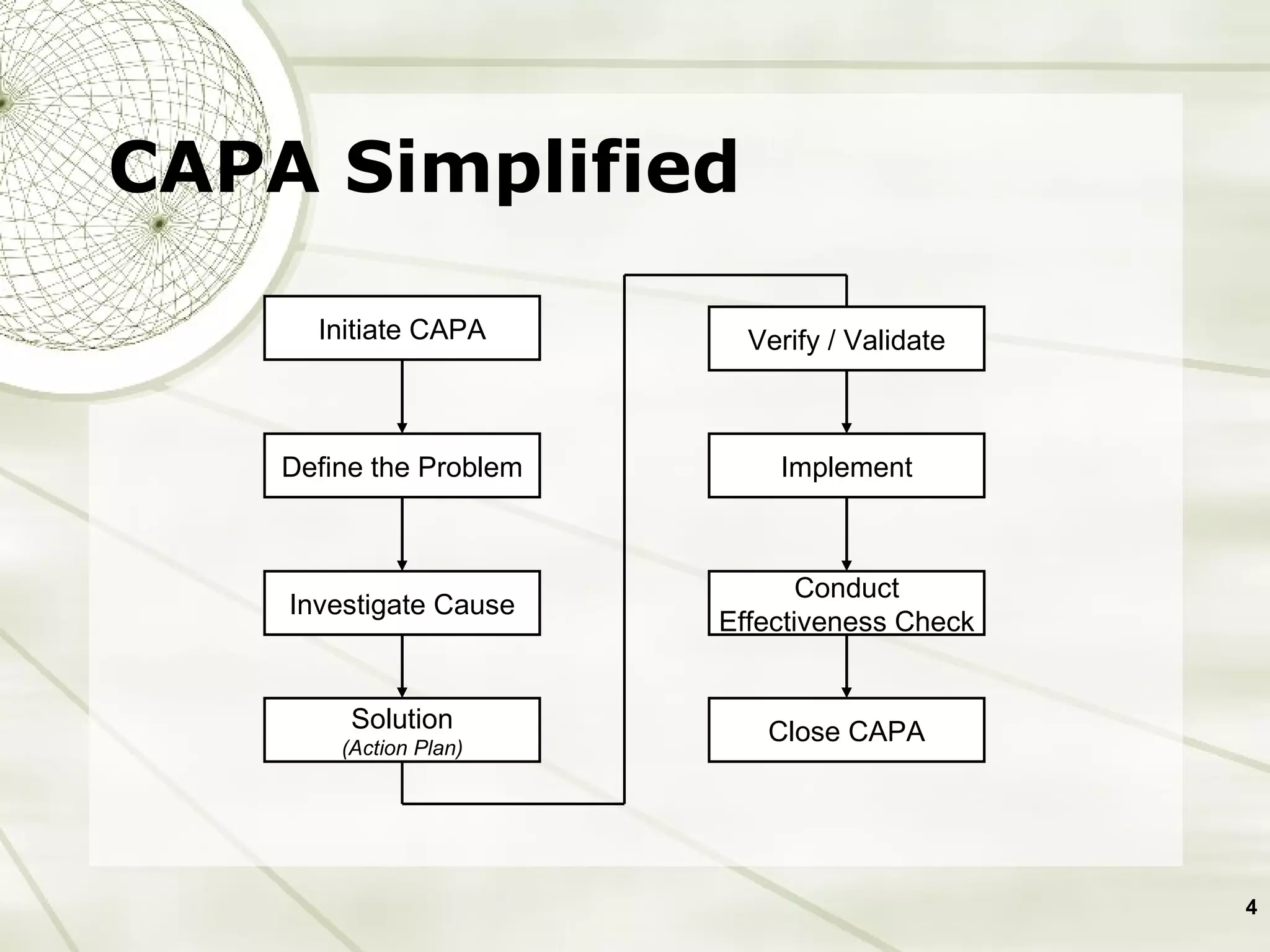
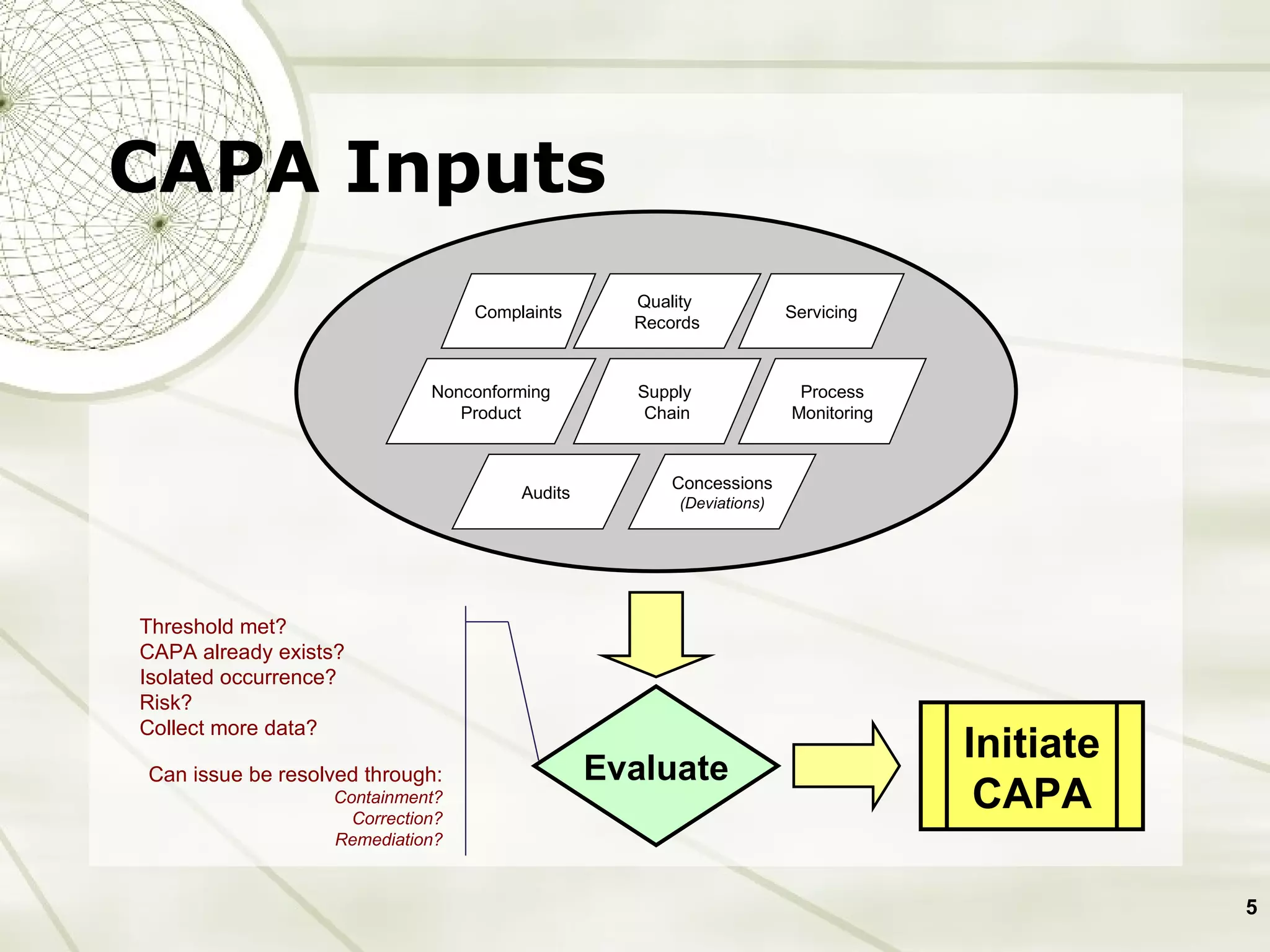
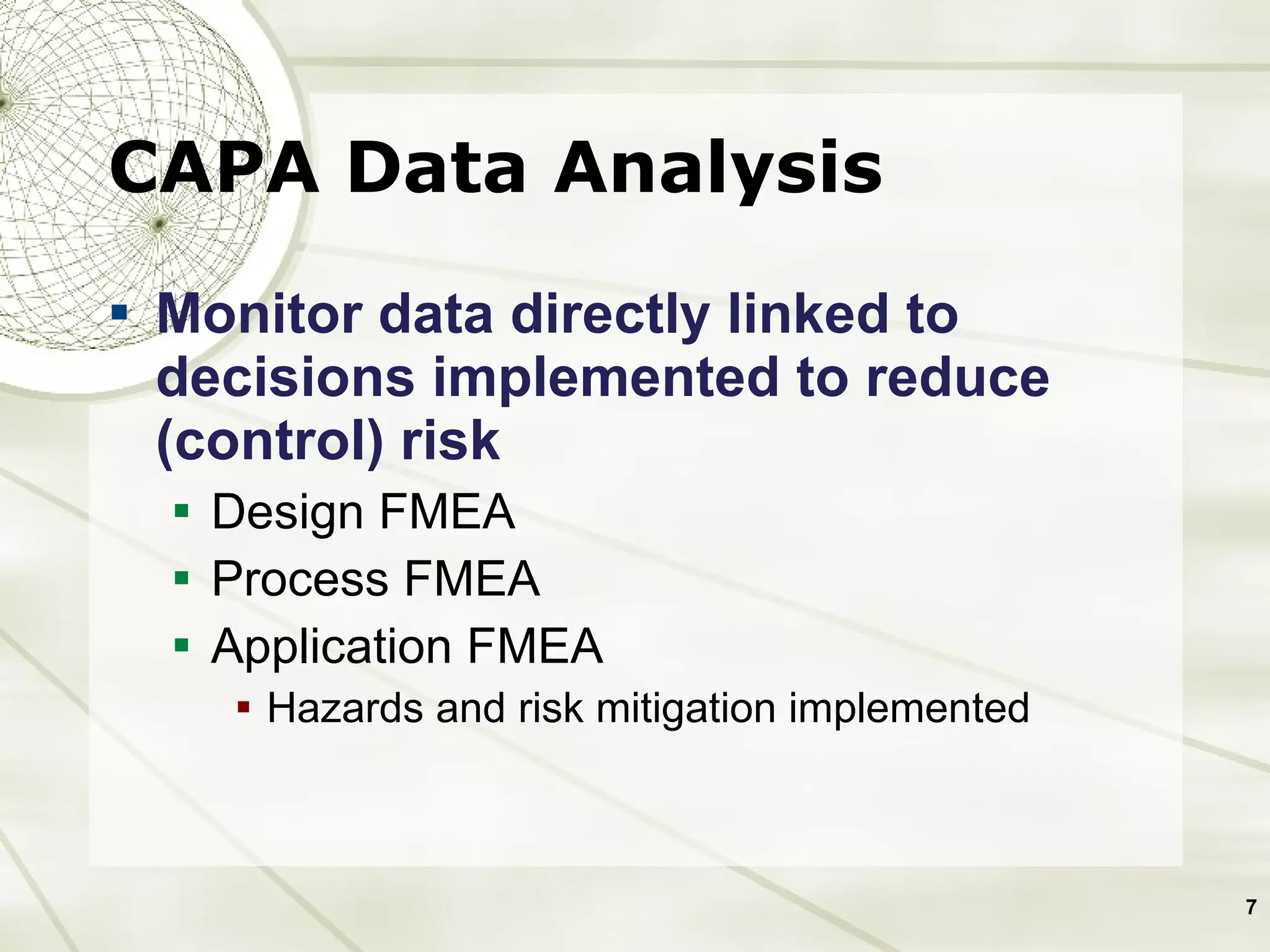
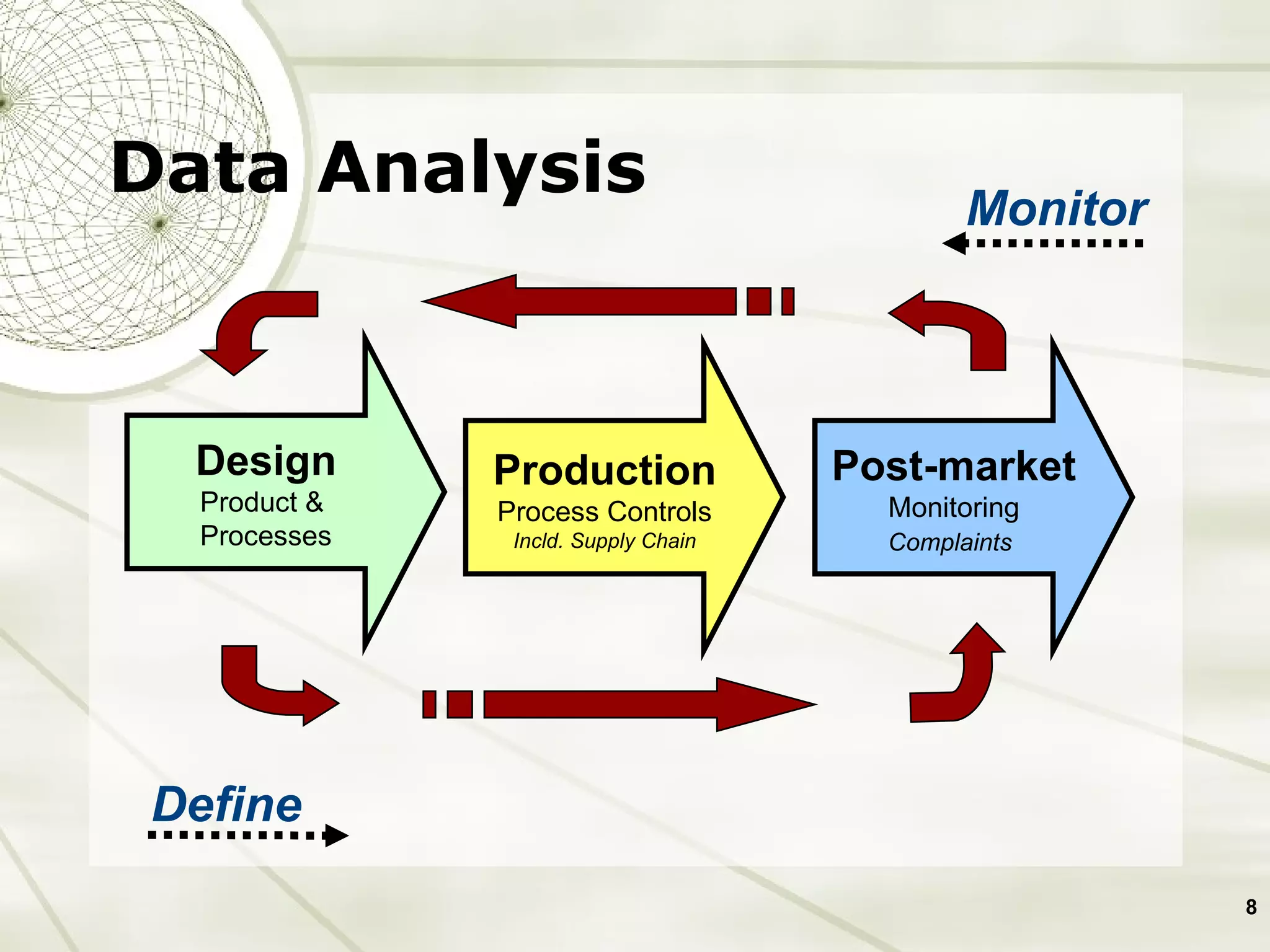
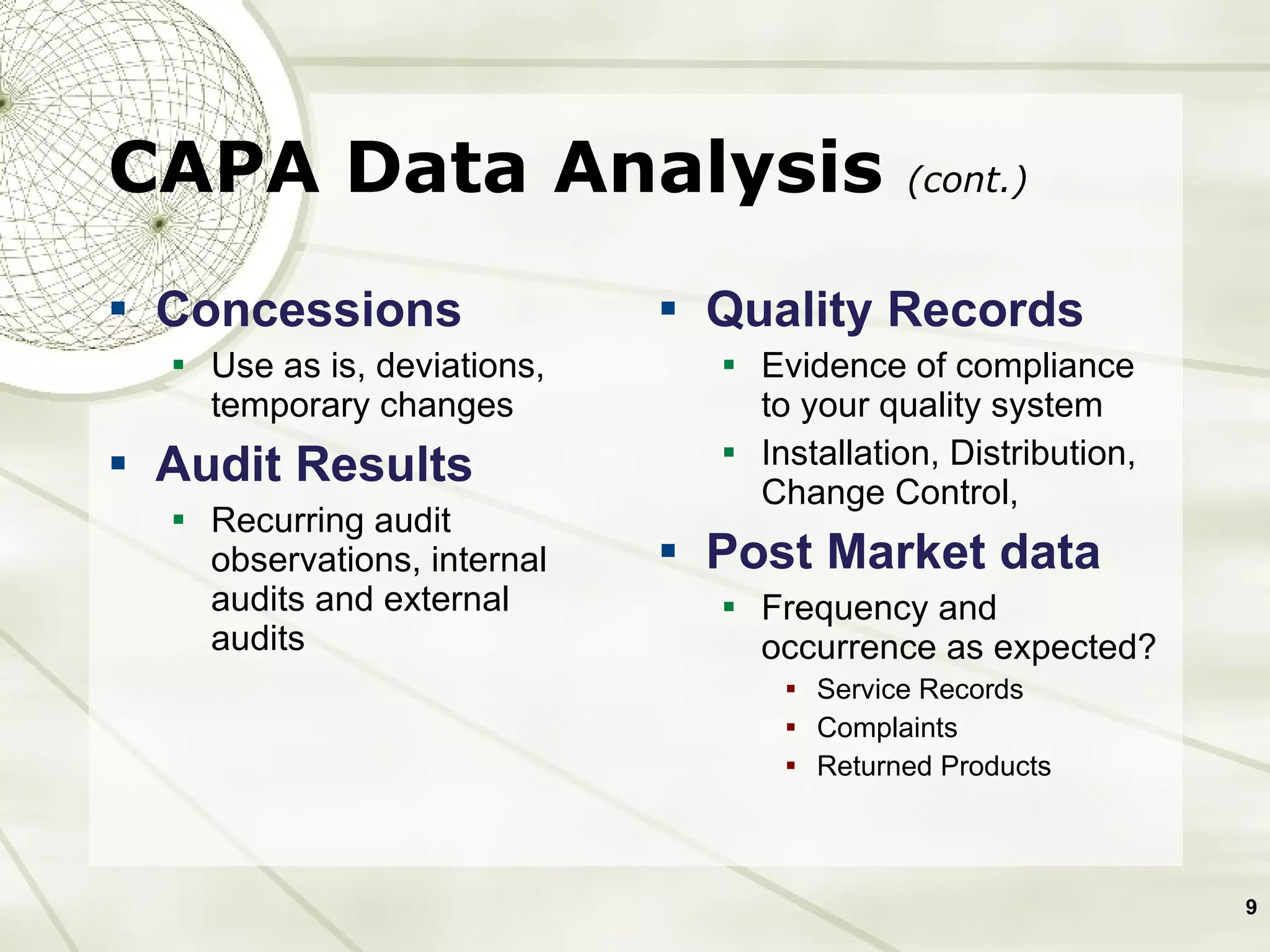
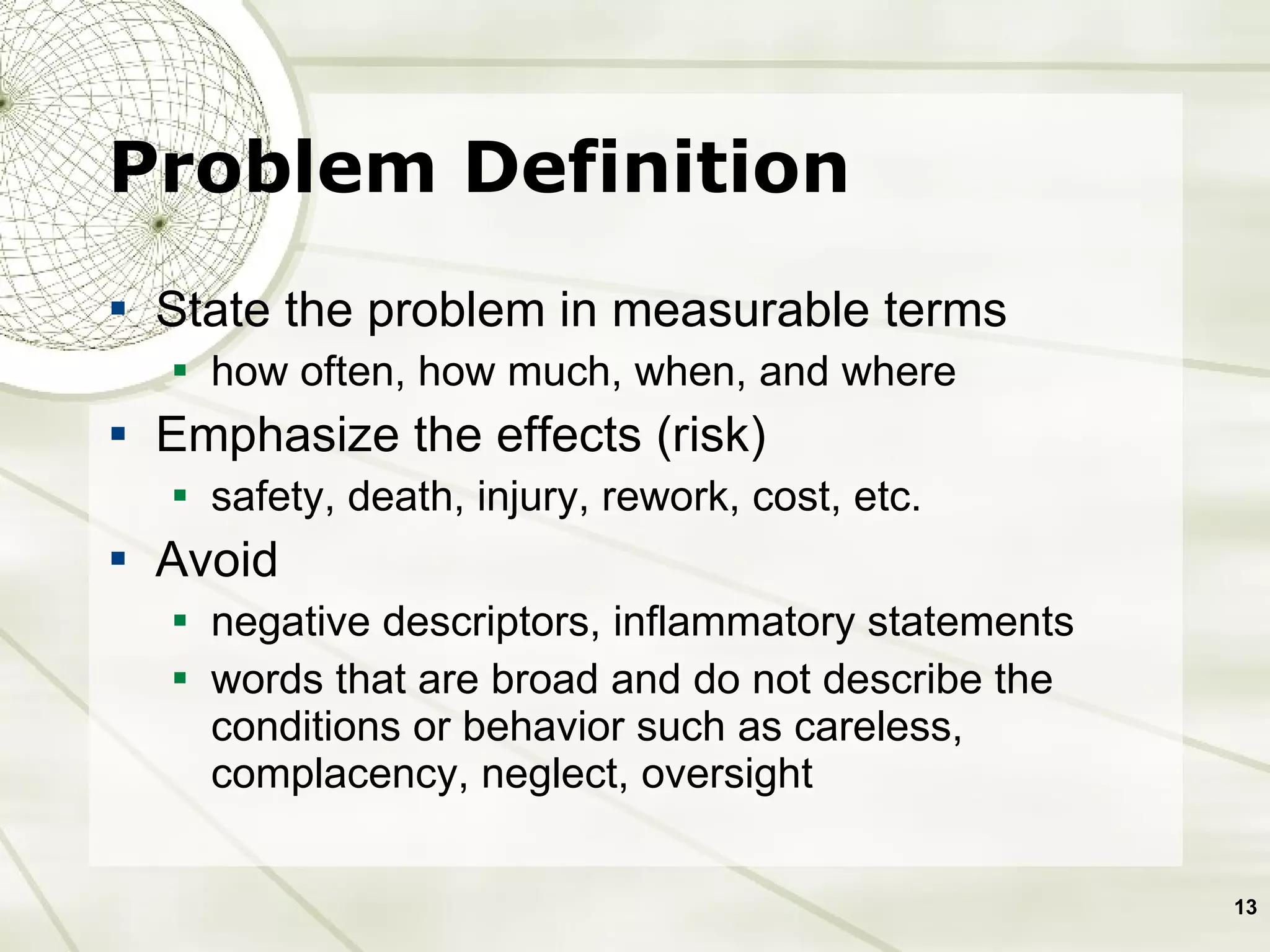
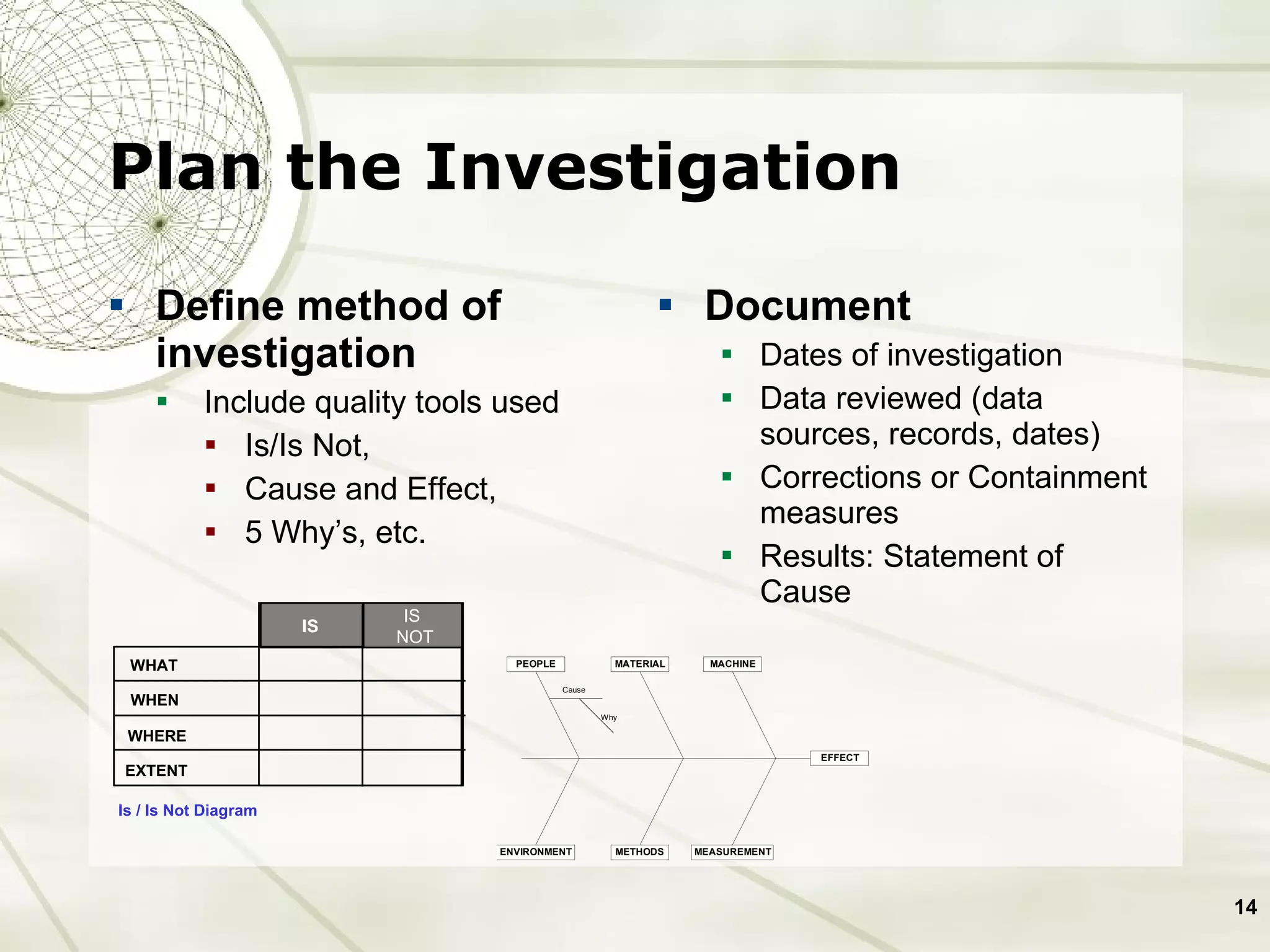
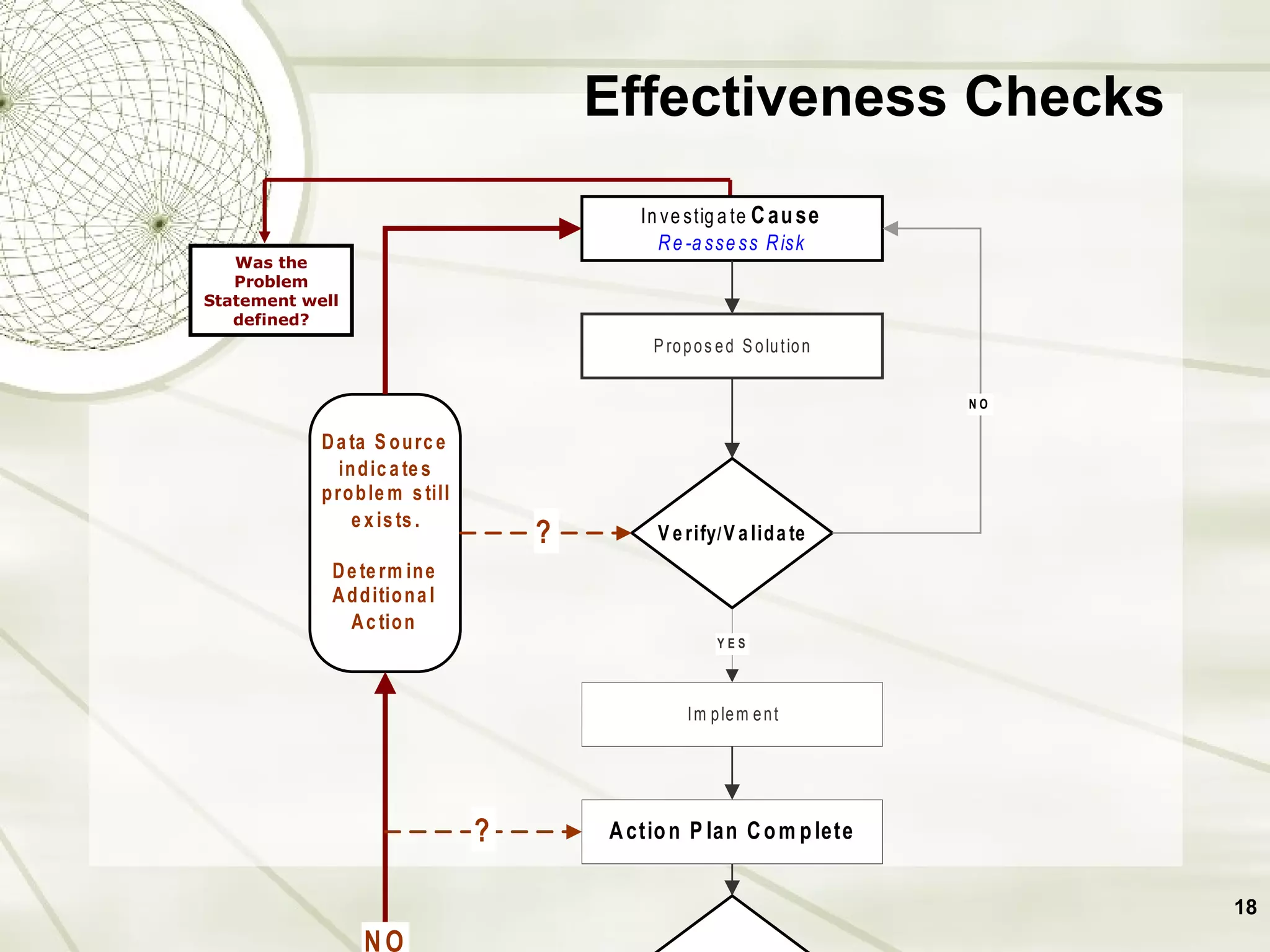
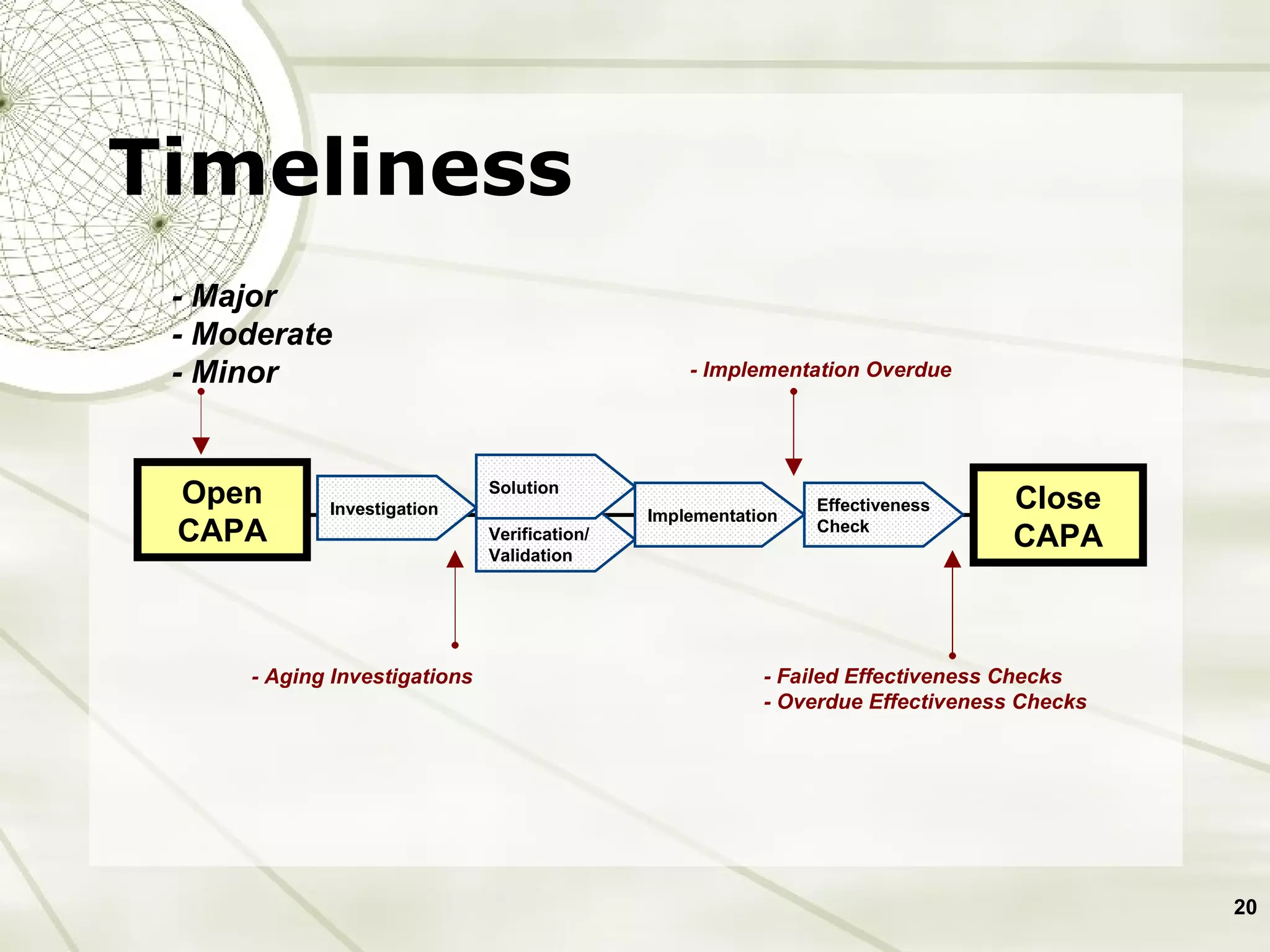
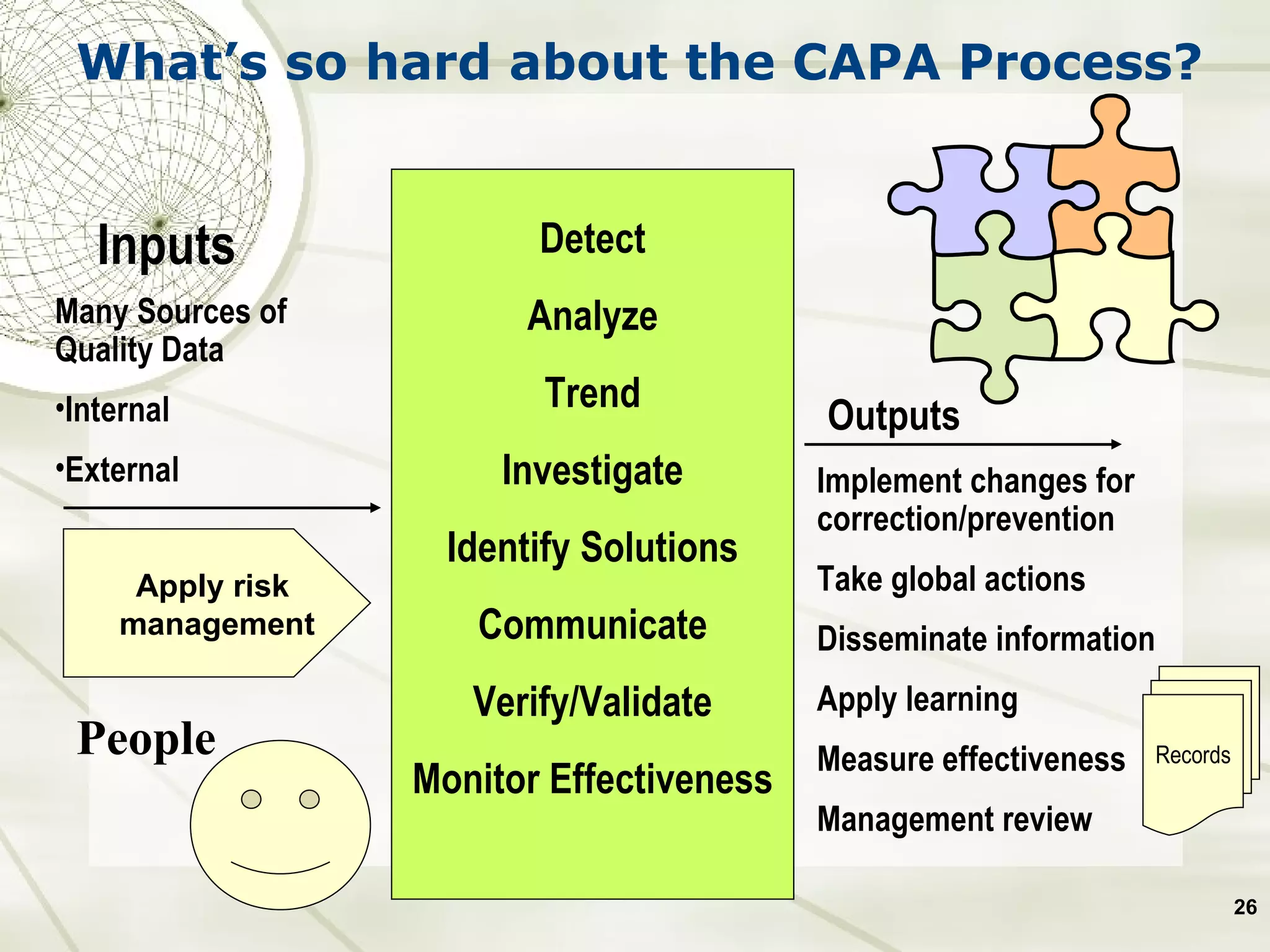
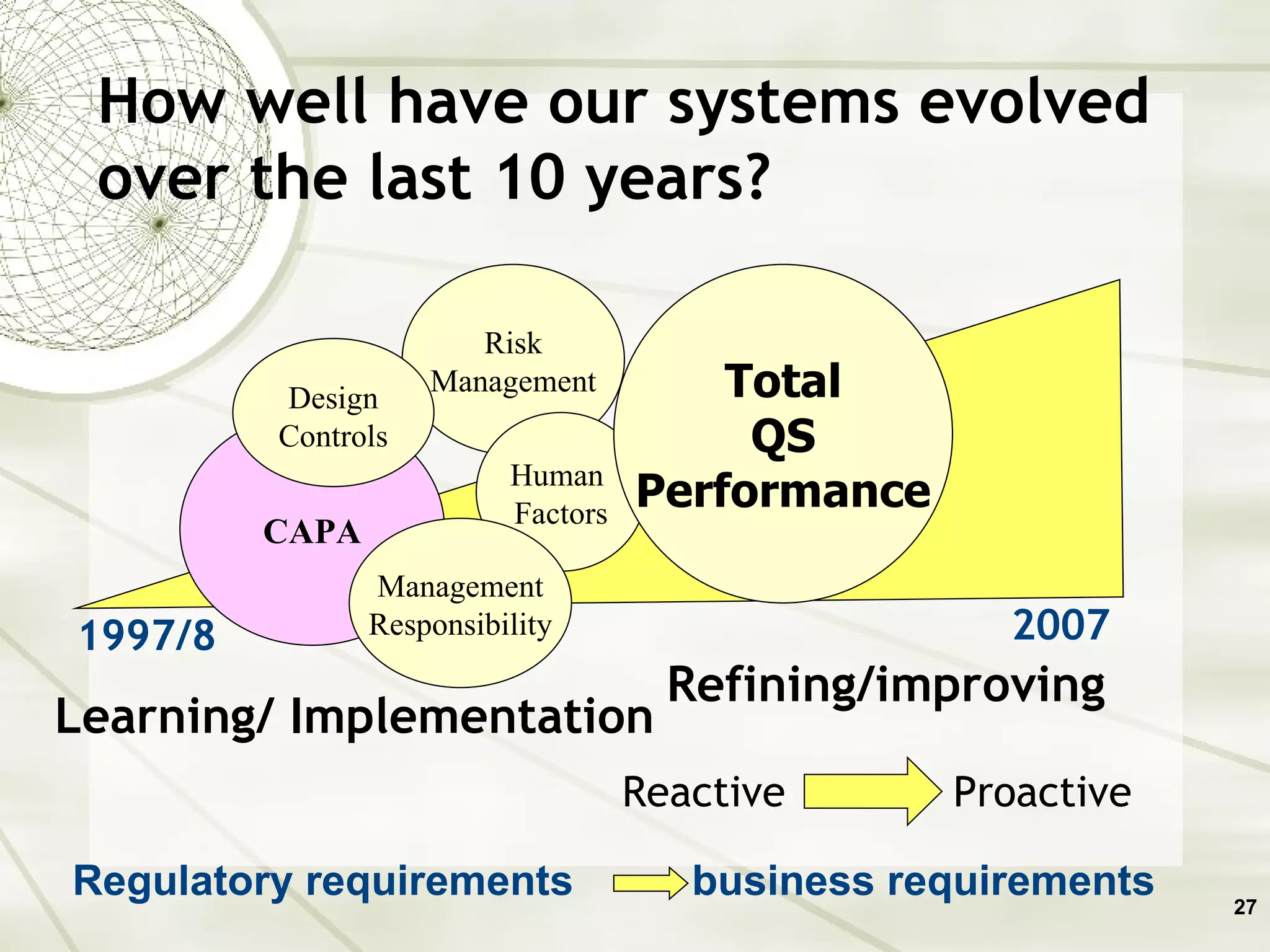
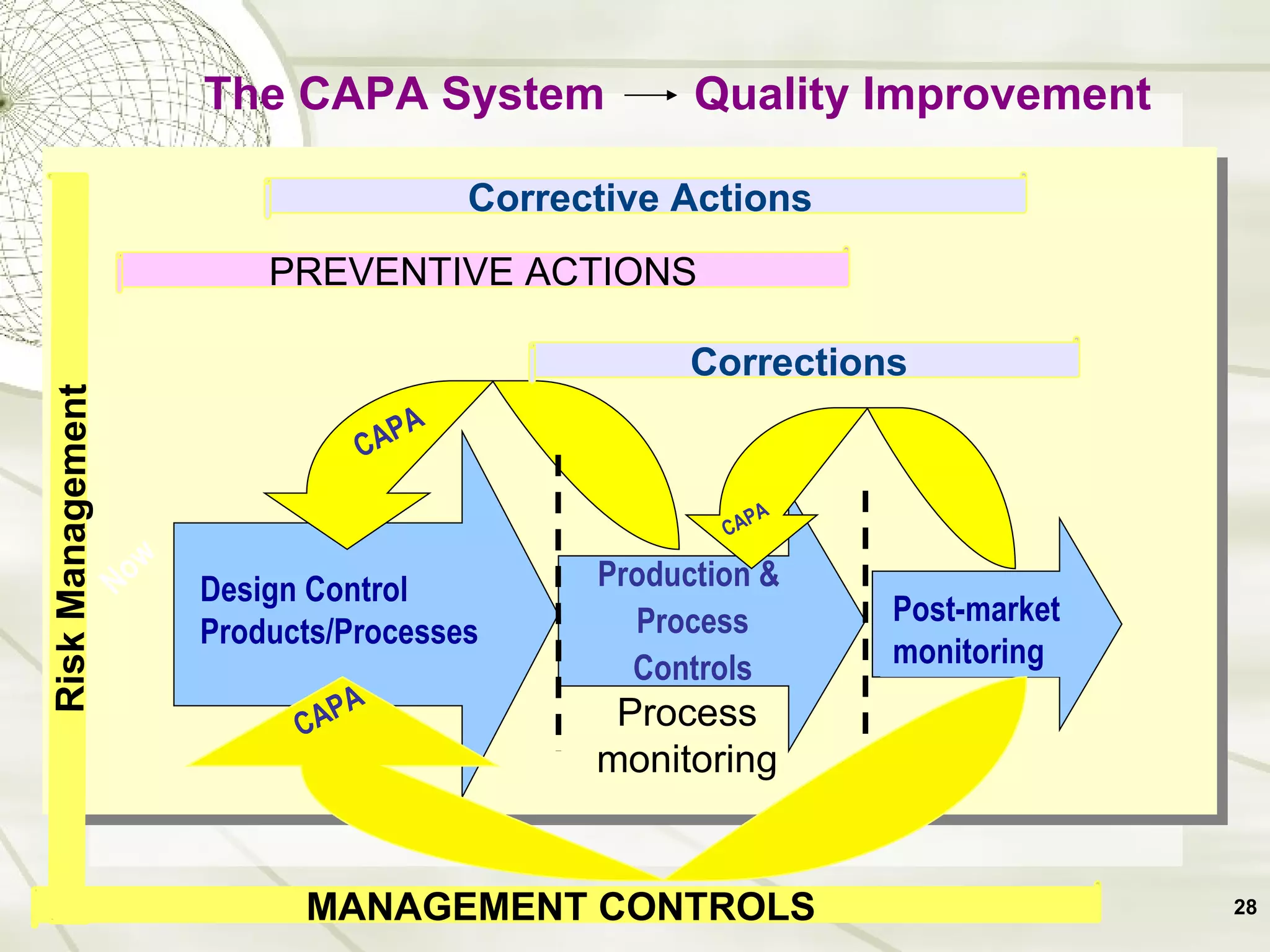
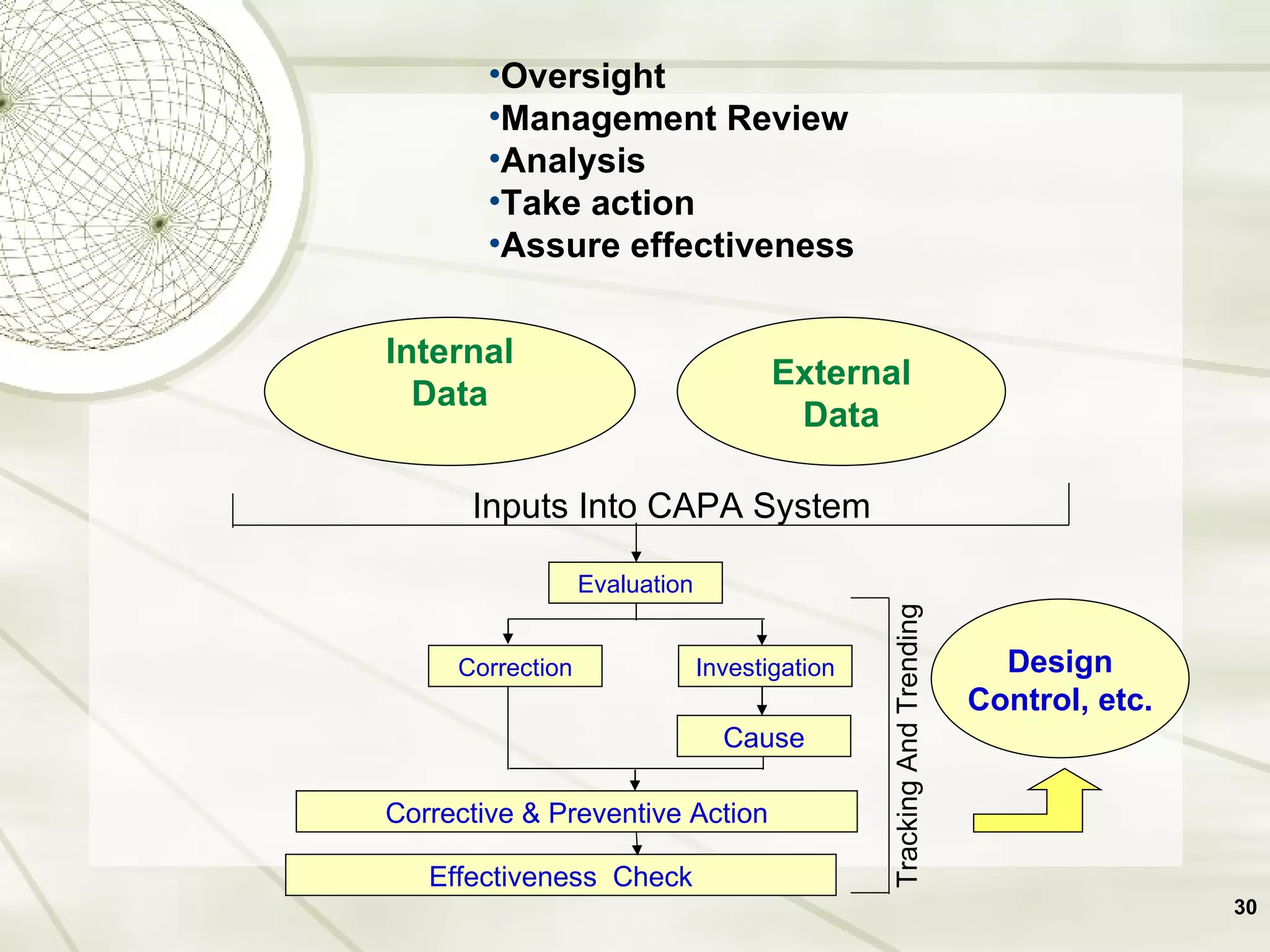
The document discusses elements of an effective Corrective and Preventive Action (CAPA) process. It outlines key aspects such as having a documented procedure, risk assessment, investigation techniques, defined action plans, effectiveness checks, and management review. The goals of a CAPA process are to determine the root cause of problems, implement corrective actions to address the cause, and verify that the actions were effective in preventing recurrence. An effective CAPA system relies on analyzing various quality data sources, taking actions to continuously improve products and processes, and demonstrating the ability to meet business and compliance needs over time.
![Implementing an Effective CAPA Process Sue Jacobs President QMS Consulting, Inc. Hoffman Estates, IL [email_address] 847.359.4456 Cecilia Kimberlin, PhD Medical Products Group VP Abbott Laboratories Abbott Park, IL [email_address] 847.937.7933](https://image.slidesharecdn.com/capa-12784377316986-phpapp01/75/Capa-1-2048.jpg)















![Thank-You ! Questions? Thoughts? Ideas? Sue Jacobs QMS Consulting, Inc. 847 359 4456 [email_address] Cecilia Kimberlin Abbott Laboratories 847 937 7933 [email_address]](https://image.slidesharecdn.com/capa-12784377316986-phpapp01/75/Capa-33-2048.jpg)