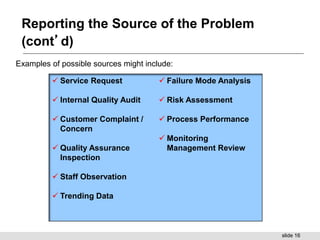
This document provides a summary of a training presentation on CAPA (corrective and preventive action). The training covers 5 learning segments: 1) CAPA general information, 2) identification of the problem, 3) evaluation, investigation and analysis of the problem, 4) development of an action plan, and 5) implementation and follow-up of the action plan. Segment 1 defines CAPA and the difference between corrective and preventive actions. It also discusses the objectives, regulations and importance of the CAPA process. Segment 2 covers reporting the source and details of a problem and determining supporting evidence. The training is intended to provide an overview of the full CAPA process.