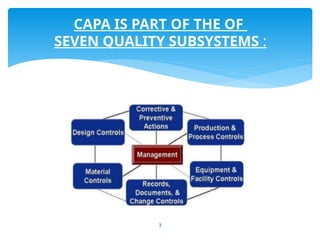
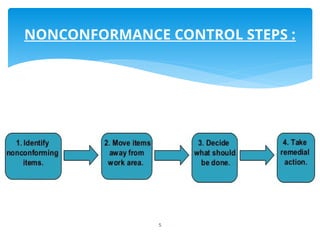
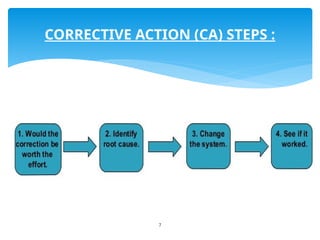
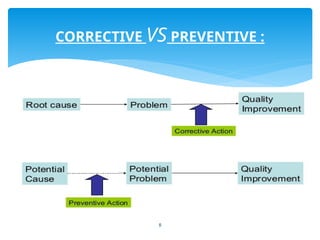
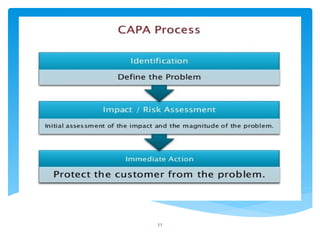
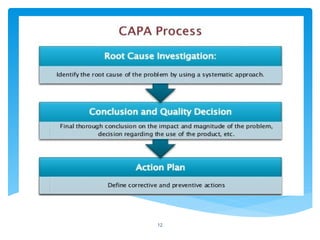
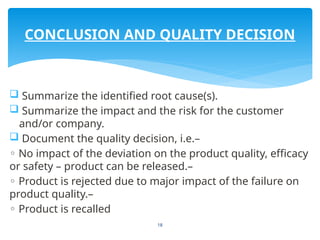
The document outlines the components and processes of Corrective and Preventive Actions (CAPA) within a quality management system, emphasizing the importance of addressing nonconformities to enhance product quality. It describes the steps involved in identifying, investigating, and implementing solutions for existing and potential quality issues. Additionally, it highlights the necessity of systematic evaluations and action plans to ensure compliance and continuous improvement in quality management.