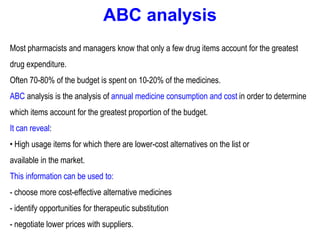
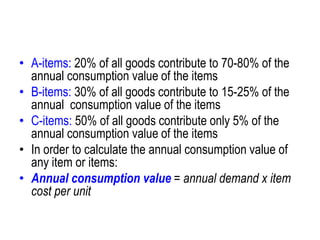
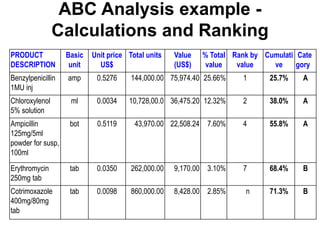
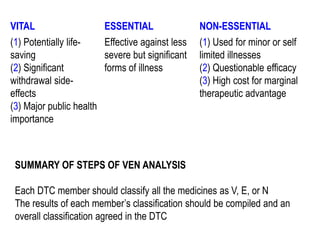
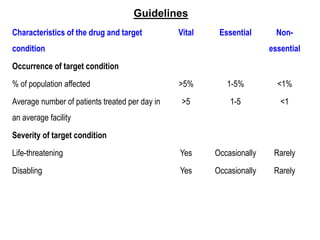
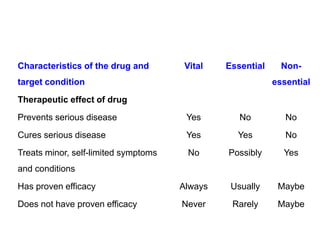
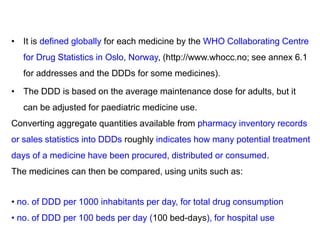
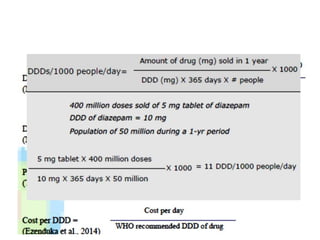
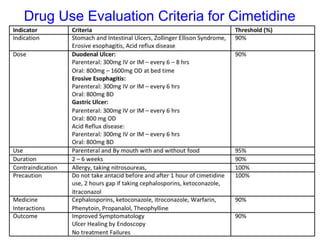
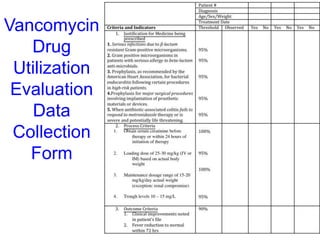
The document outlines the history and evolution of drug use evaluation (DUE) and drug utilization review (DUR) in healthcare, emphasizing the importance of evaluating medication use to enhance patient safety and outcomes. It discusses methodologies for assessing drug utilization, including performance improvement methods and various analyses to monitor prescribing patterns and safety issues. The role of pharmacists in medication use evaluation processes is highlighted, along with strategies for ensuring rational drug use and addressing issues like polypharmacy.