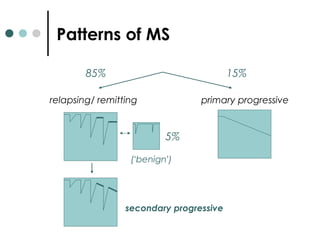
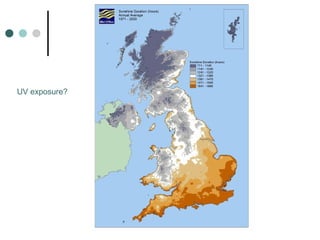
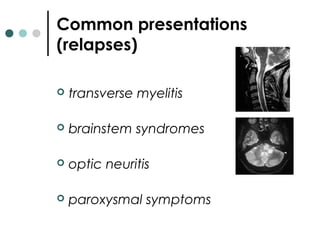
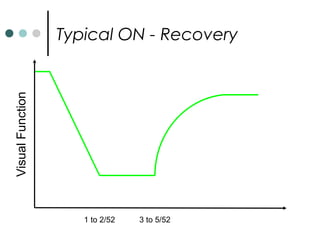
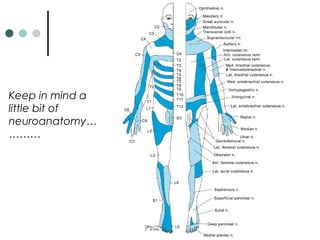
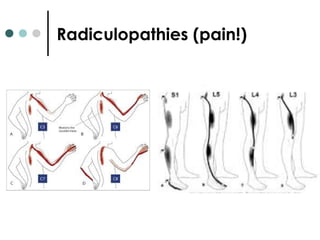
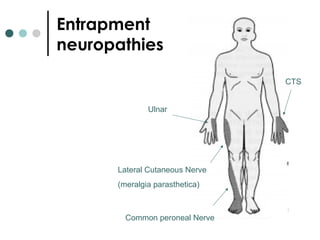
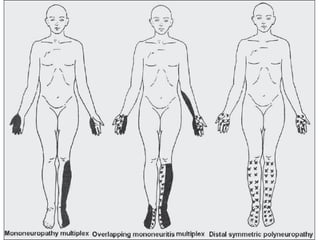
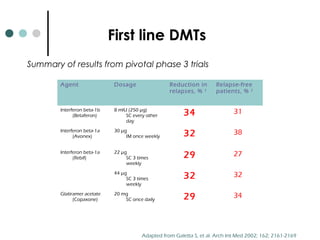
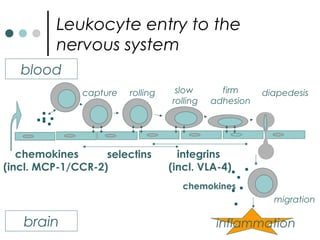
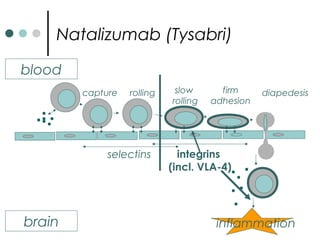
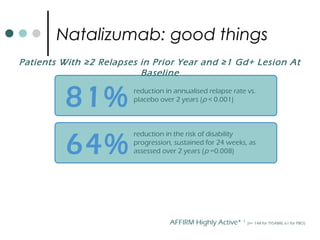
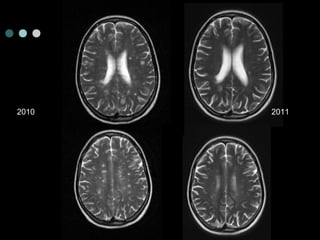
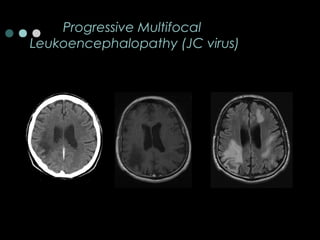
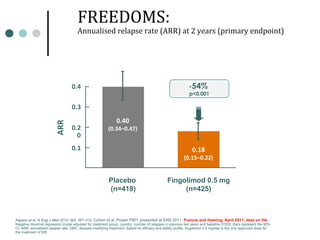
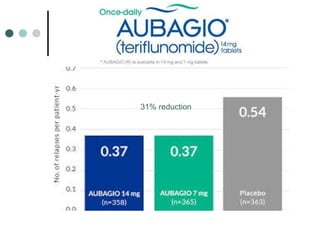
This document provides information about multiple sclerosis (MS) and sensory symptoms that may indicate MS. It begins with statistics on the prevalence of MS, noting it is most common in young people and more prevalent in women. Typical presentations of MS include transverse myelitis, brainstem syndromes, optic neuritis, and paroxysmal symptoms. Common sensory symptoms of MS are described, such as ascending numbness in the feet or bilateral hand numbness. The document outlines current disease-modifying therapies for MS including interferon-beta medications and glatiramer acetate. It discusses new oral and infusion therapies in development like fingolimod, dimethyl fumarate, and alemtuzumab.