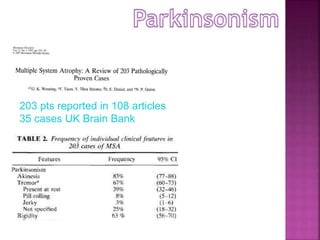
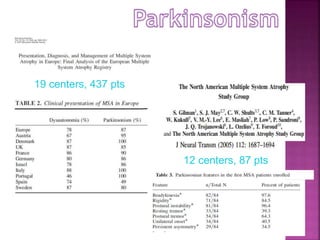
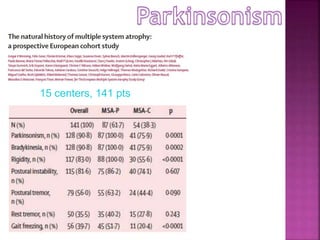
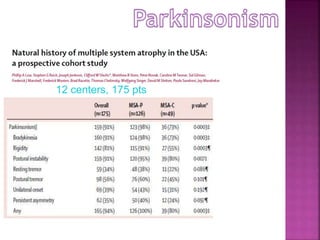
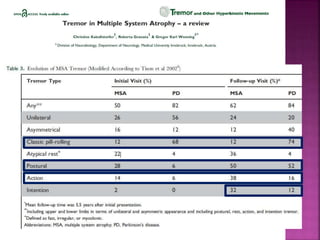
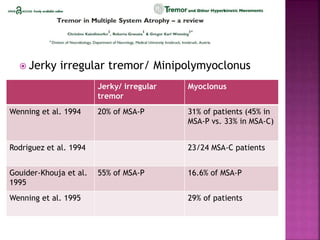
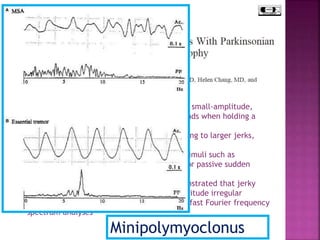
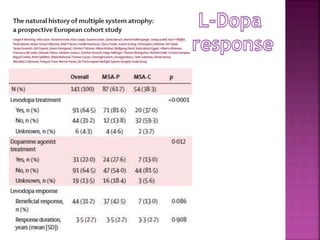
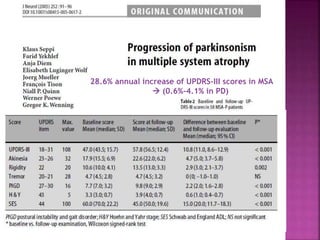
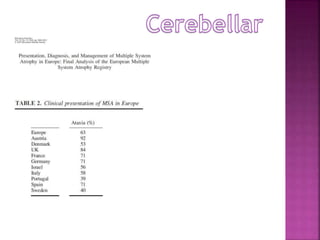
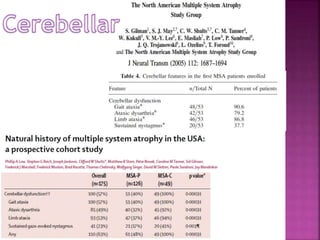
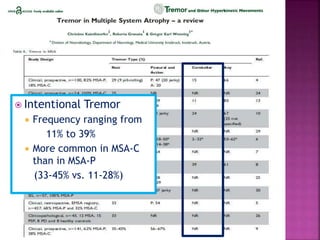
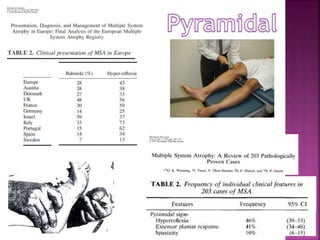
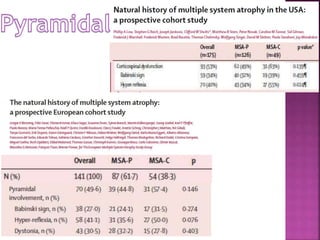
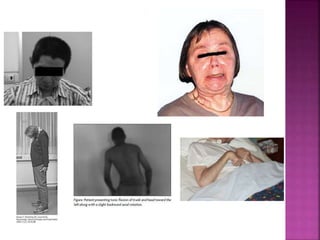
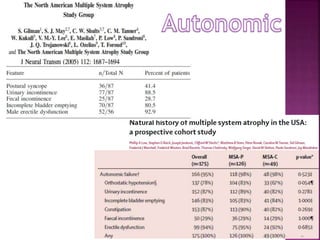
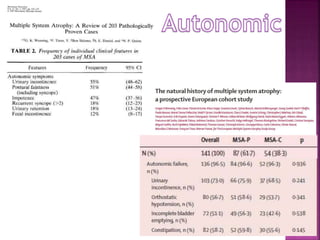
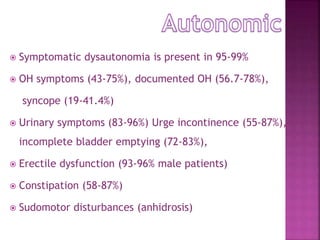
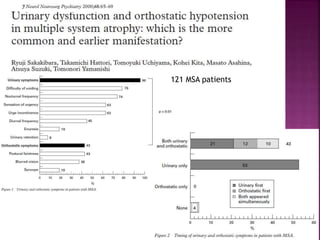
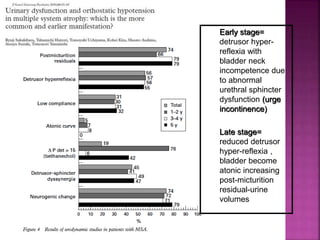
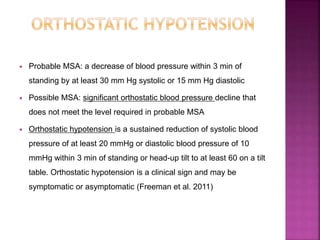
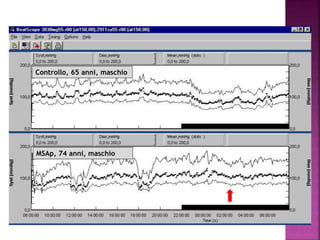
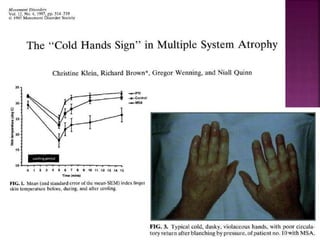
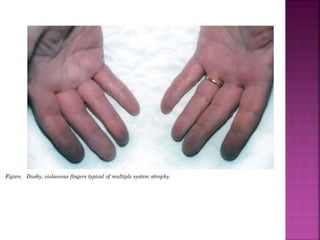
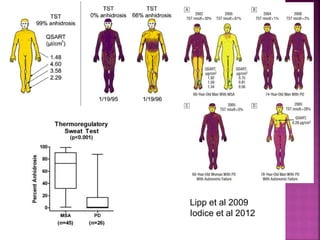
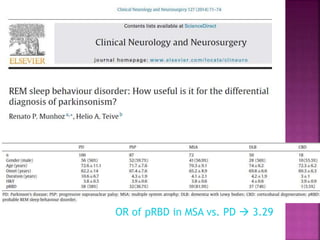
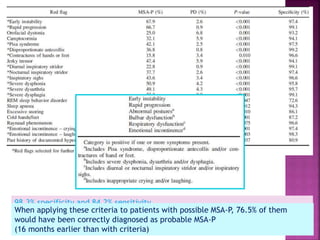
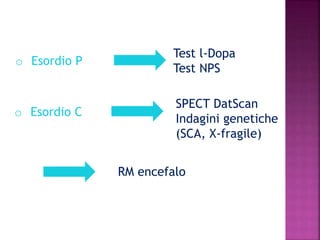
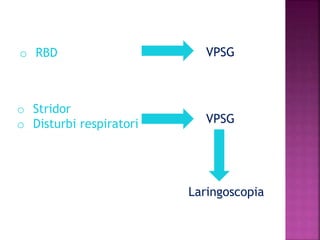
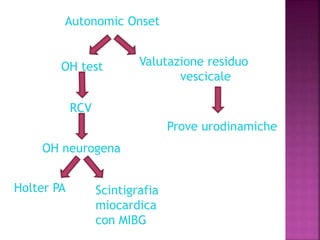
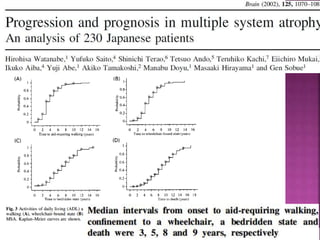
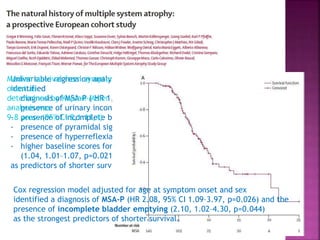
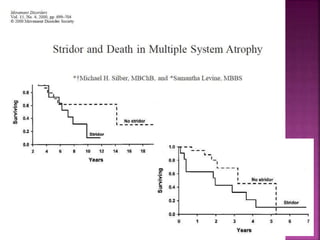
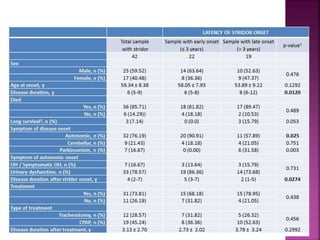
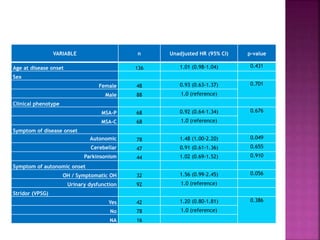
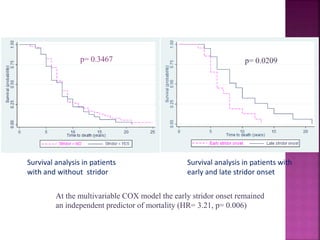
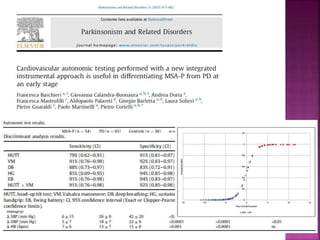
This document summarizes information about multiple system atrophy (MSA). MSA is a neurodegenerative disorder characterized by autonomic failure and motor symptoms. It has an estimated incidence of 0.6-0.7 per 100,000 people per year. Common features include parkinsonism in at least 90% of patients, orthostatic hypotension, and urinary dysfunction in over 80% of cases. The document discusses the types of tremors seen in MSA, respiratory issues like stridor, and prognostic factors like the presence of urinary incontinence which are associated with shorter survival times.