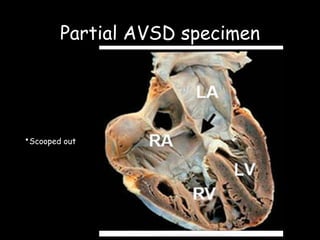
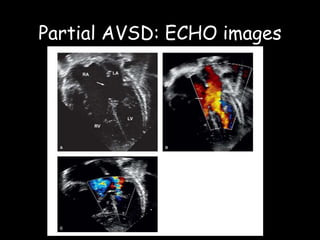
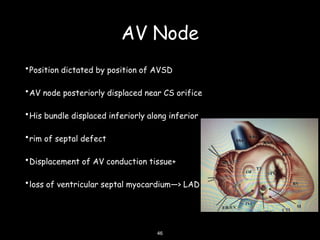
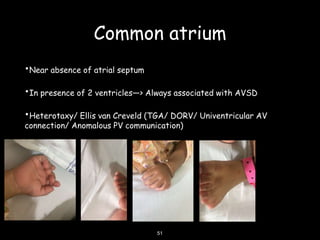
This document discusses atrioventricular septal defects (AVSDs), including their embryogenesis, classification, clinical features, imaging, and management. It describes the spectrum of AVSDs from partial to complete. Partial AVSDs involve a primum atrial septal defect with a cleft in the mitral valve. Complete AVSDs have a large ventricular septal defect with a common atrioventricular valve. Imaging like echocardiography is important for evaluating the anatomy and determining appropriate treatment, which ranges from observation to surgical repair.