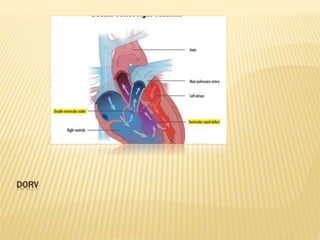
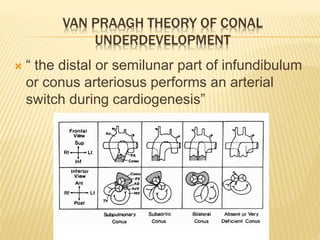
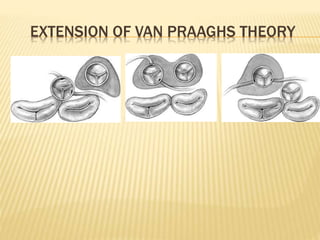
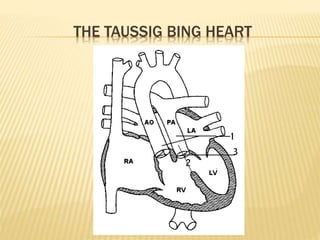
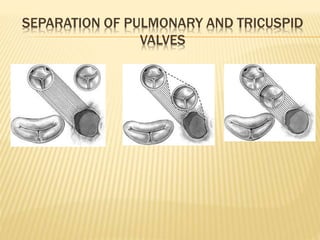
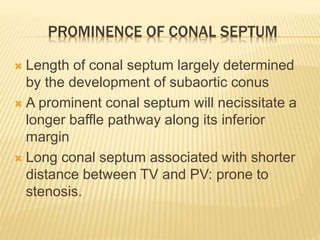
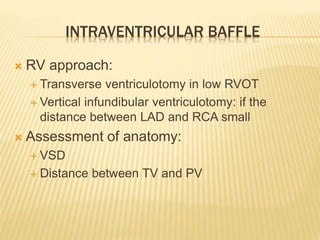
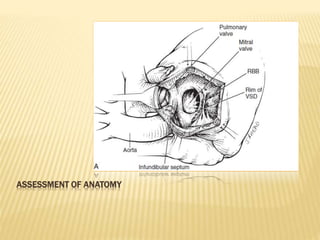
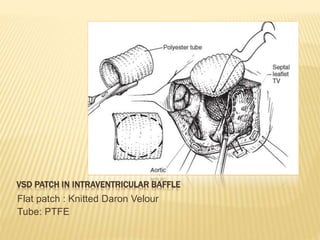
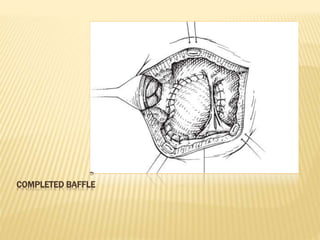
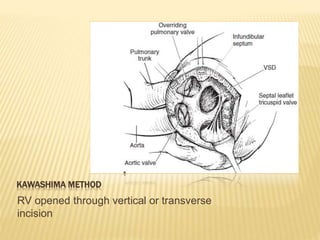
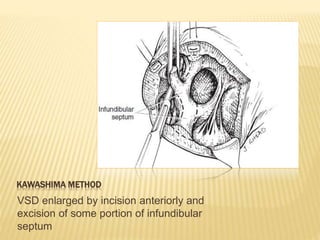
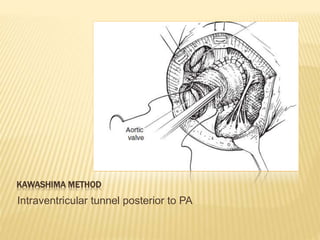
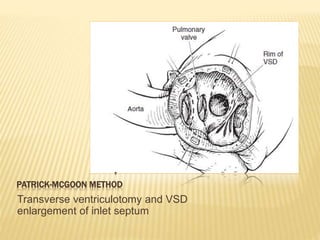
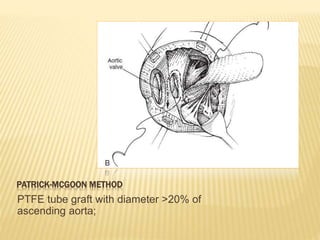
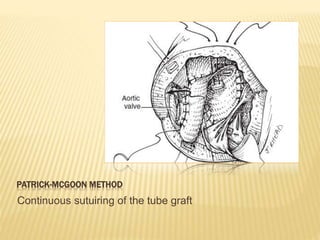
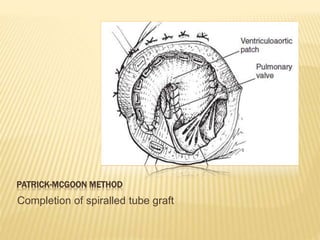
Double outlet right ventricle (DORV) was first described in 1793 and involves both great arteries arising wholly or partially from the right ventricle. The condition involves abnormalities in the development of the conus arteriosus. Repair techniques depend on factors such as the location of the ventricular septal defect and relationship between the tricuspid and pulmonary valves. Common repair methods include an intraventricular baffle technique or arterial switch operation with VSD closure for Taussig-Bing heart. Early survival rates for repair are over 95% however reoperation may be needed for baffle leaks or obstructions.