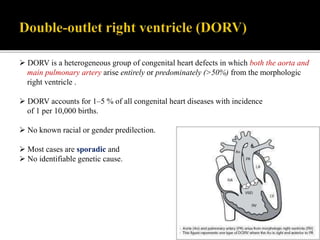
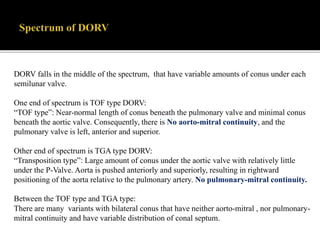
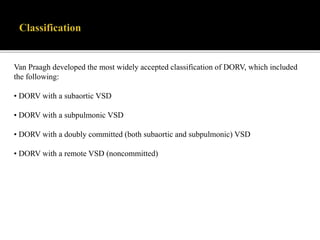
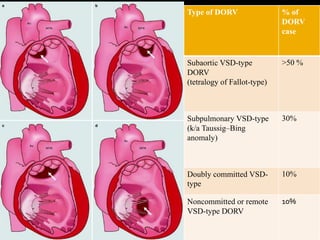
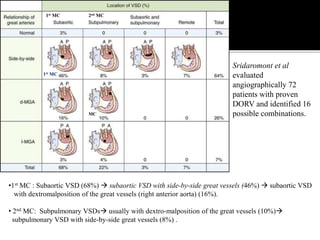
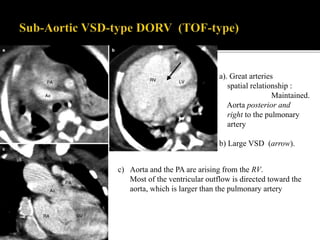
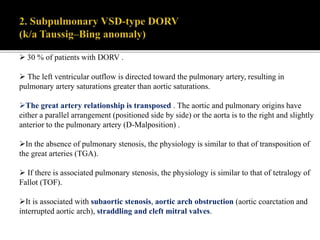
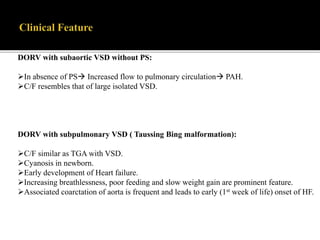
DORV is a congenital heart defect where both the aorta and pulmonary artery arise from the right ventricle. There are several types of DORV based on the location of the ventricular septal defect and the spatial relationship of the great arteries. The most common type is subaortic VSD, present in over 50% of DORV cases. Patients typically present in the first month of life with symptoms depending on the specific anatomy such as cyanosis, heart failure, or hypoxic spells. Treatment involves palliative procedures in infancy such as shunts, with definitive repair later involving procedures to redirect blood flow such as arterial switch operation or Fontan procedure.