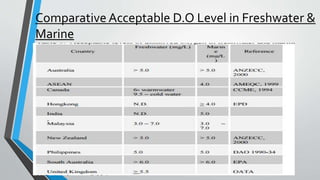
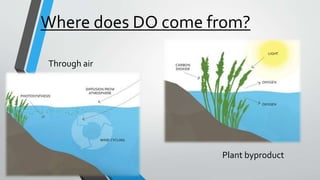
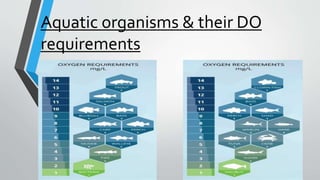
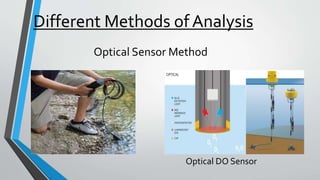
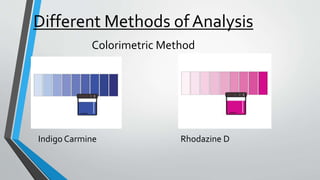
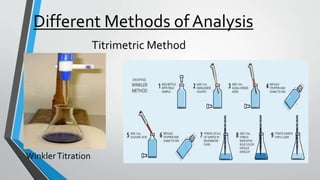
This document discusses dissolved oxygen (DO) levels in drinking water and their importance for aquatic life. It provides a table comparing drinking water quality standards in different countries that includes testing for microbiological, chemical, physical, and radiological parameters. The document discusses the sources and importance of DO, how aquatic organisms rely on it, and consequences of unusual DO levels. It also outlines several common methods for analyzing DO levels, including optical, electrochemical, colorimetric, and titrimetric methods.