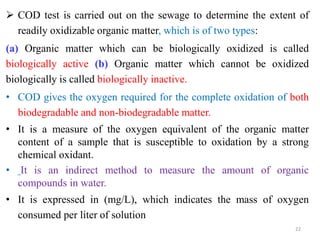
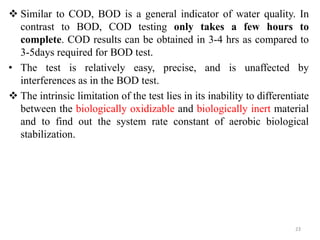
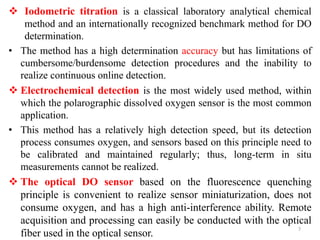
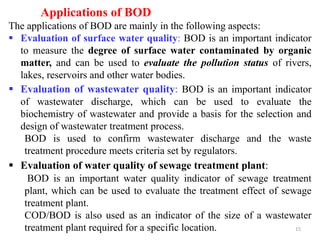
The document discusses the measurement of dissolved oxygen (DO) and biochemical oxygen demand (BOD), emphasizing their importance for aquatic life and water quality assessment. It details methods for measuring DO such as the Winkler method and the use of probes, as well as the impact of factors like temperature and sewage on oxygen levels. Additionally, it explains the chemical oxygen demand (COD) as a measure of water pollution and treatment efficiency, highlighting both BOD and COD's significance in environmental monitoring.






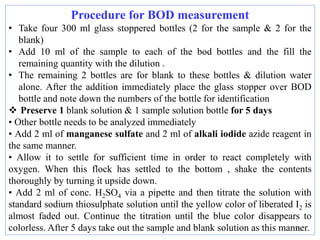
![Concept: O2 measurement depends on the fact that O2 oxidizes Mn2+
under alkaline conditions:
Mn2+ + 2OH + ½O2 MnO2 + H2O
MnO2 is capable of oxidizing iodide (I (to free iodine (I2) under
acidic conditions:
MnO2 + 2 I + 4H+ Mn2+ + I2 + H2O
The amount of free iodine released is equivalent to the DO.
BOD5 (mg/L) = [(DOInitial - DOFinal )] x 300/sample volume](https://image.slidesharecdn.com/chap4bodandcod-241221160416-8e6fdfc9/85/Chap-4-BOD-and-CODnoonpodefgpnmopoon-pdf-11-320.jpg)



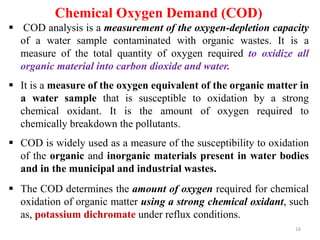

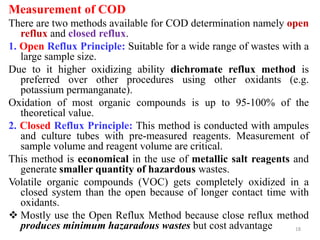


![21
COD is given by
COD (mg O2 /L) = [(A-B) × M] ×8000/ V sample
Where: A = volume of FAS used for blank (mL). B = volume of FAS used
for sample (mL), M = molarity of FAS, 8000 = milli equivalent weight of
oxygen (8) ×1000 mL/L
Water with high COD typically contains high levels of decaying
plant matter, human waste, or industrial effluent.](https://image.slidesharecdn.com/chap4bodandcod-241221160416-8e6fdfc9/85/Chap-4-BOD-and-CODnoonpodefgpnmopoon-pdf-21-320.jpg)