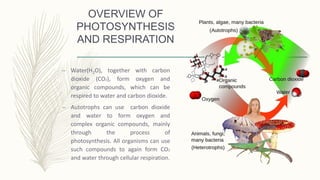
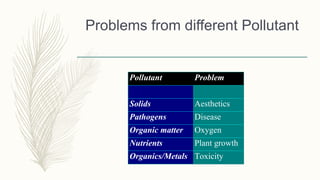
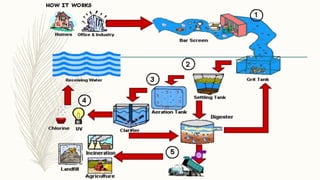
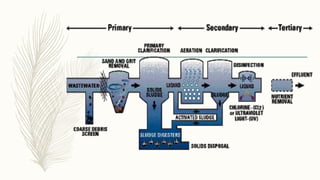
This document discusses key concepts related to waste water treatment including biochemical oxygen demand (BOD), chemical oxygen demand (COD), and dissolved oxygen (DO). BOD measures the amount of oxygen required by microorganisms to break down organic matter in water. COD determines the oxygen required to oxidize organic compounds. DO refers to oxygen dissolved in water that aquatic life requires. The document outlines typical values and measurement methods for BOD, COD and DO in waste and natural waters. It also describes the nature of waste water pollutants and an overview of waste water treatment processes.