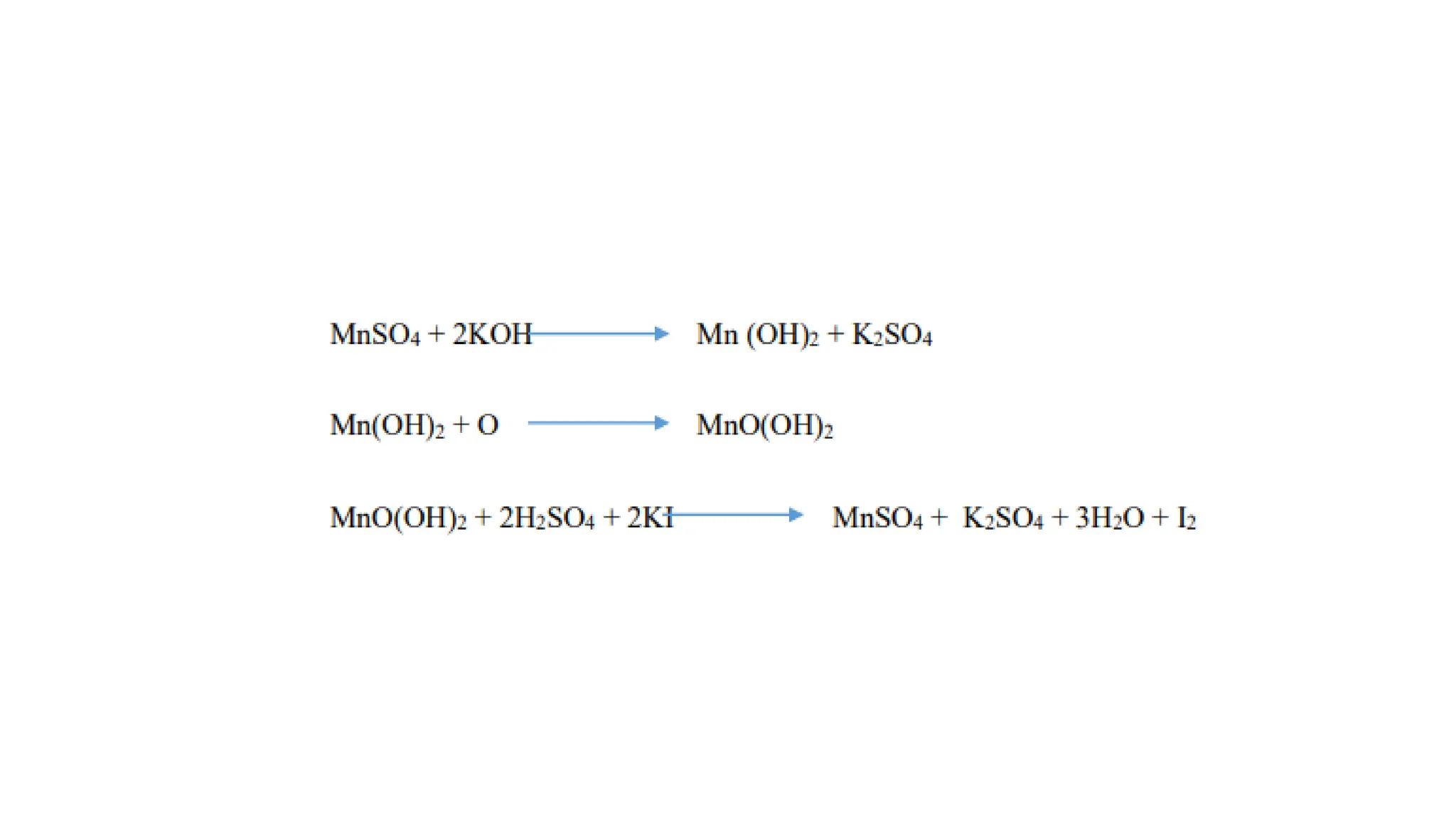
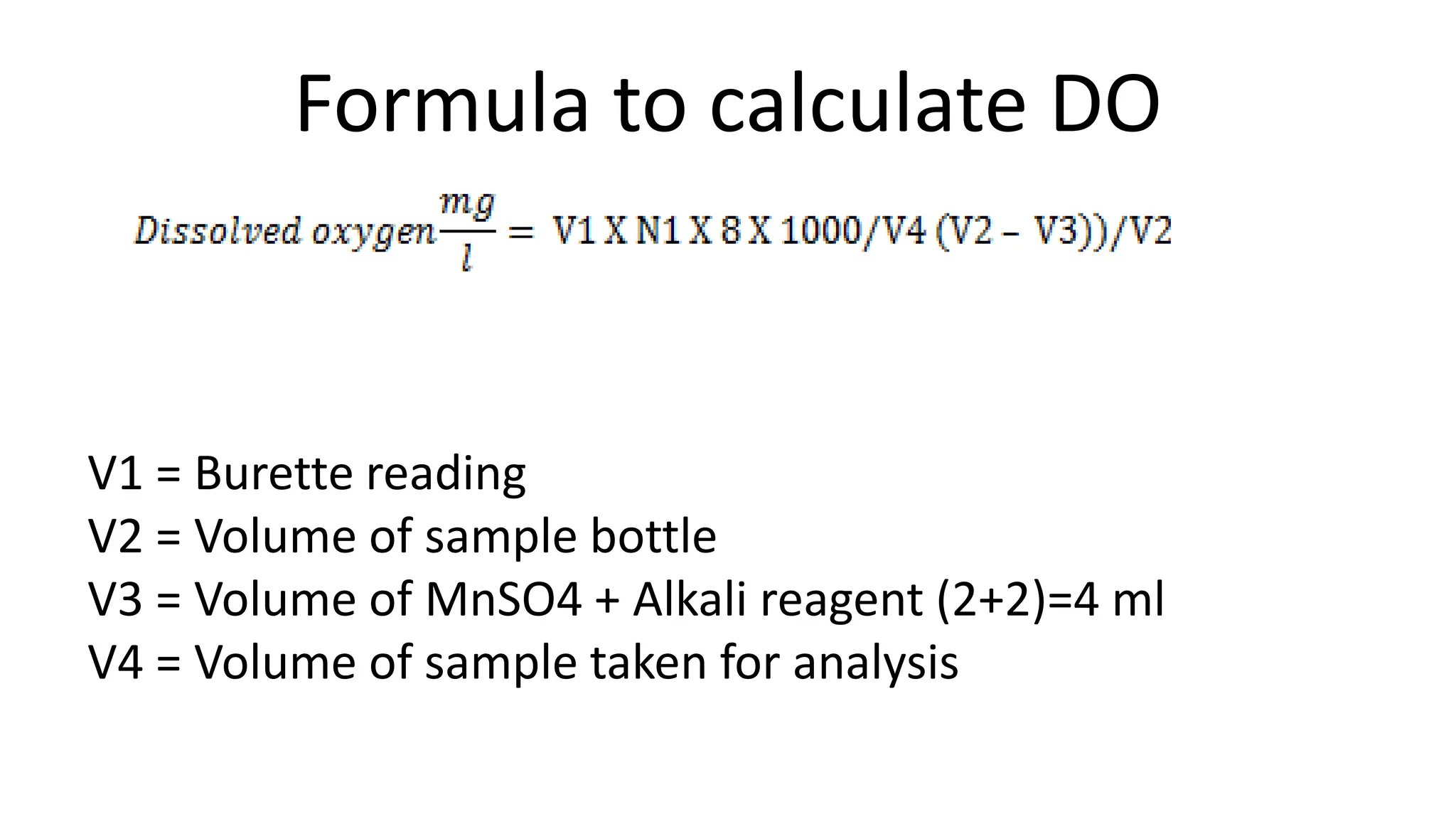
The document outlines methods for estimating dissolved oxygen (DO) levels in water, emphasizing its significance for aquatic life and water quality. It details two common methods for DO measurement: the iodometric method and the membrane electrode procedure, including necessary chemicals and apparatus. The document also provides a step-by-step procedure for the iodometric method, including preparation of reagents and the titration process.