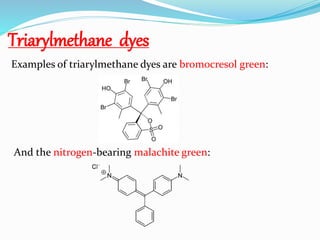
This document discusses triphenylmethane, which is a colorless solid hydrocarbon that serves as the basic skeleton for many synthetic dyes called triarylmethane dyes. It can be synthesized through various reactions including the Friedel-Crafts reaction of benzene and chloroform. As a triarylmethane, it is more acidic than other hydrocarbons due to delocalization of charge over three phenyl rings. Examples provided of triarylmethane dyes include bromocresol green and malachite green.