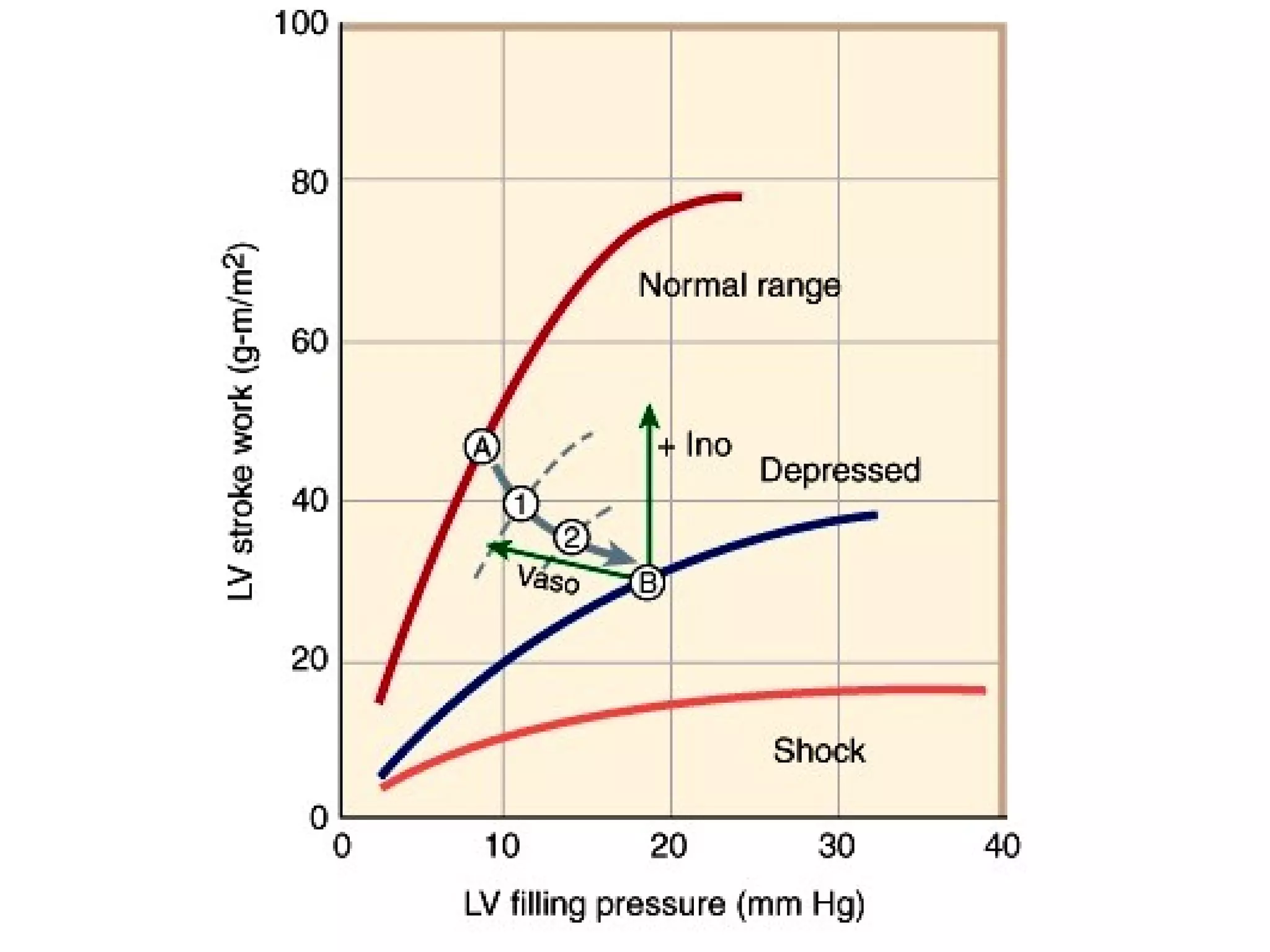
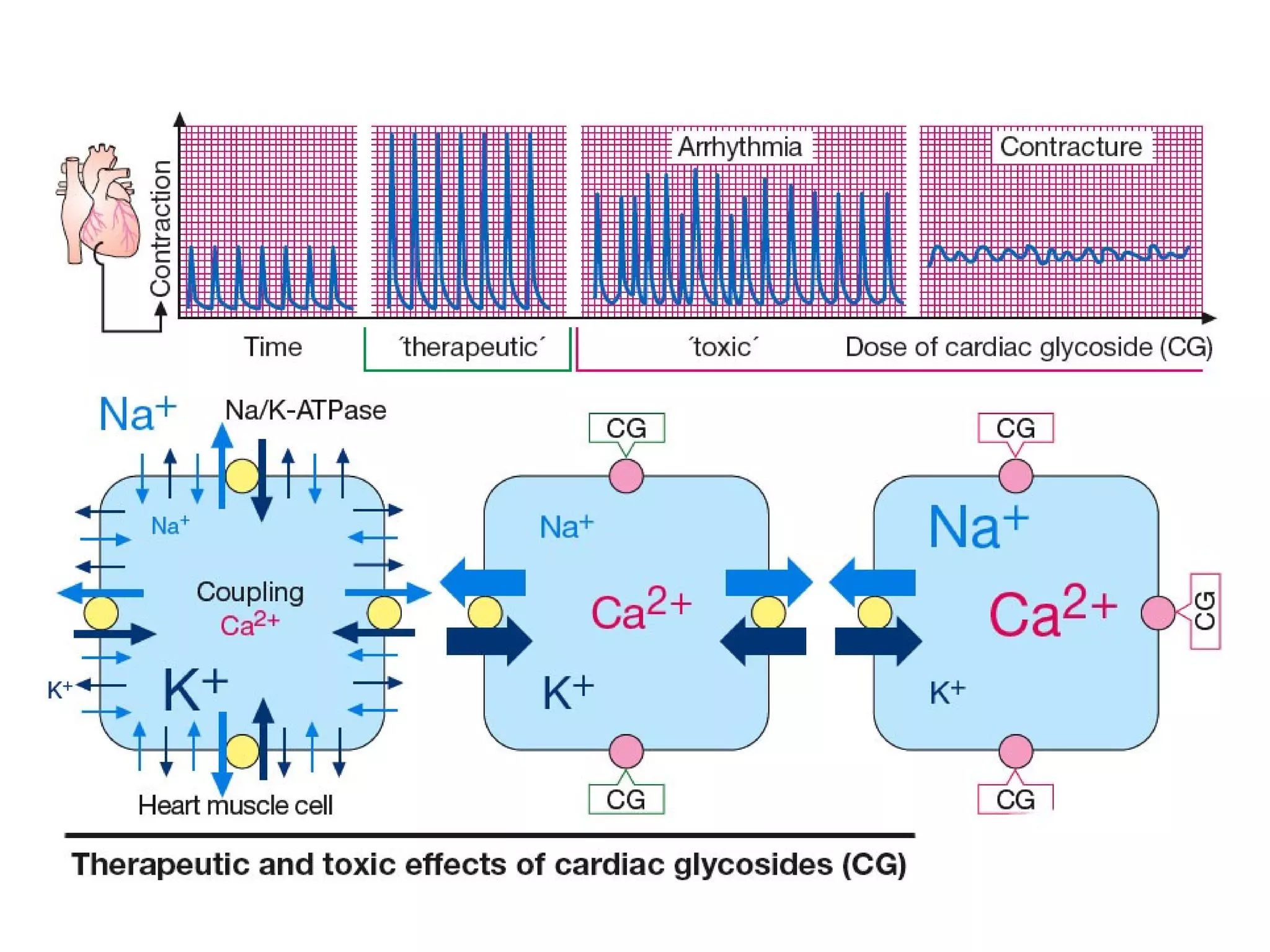
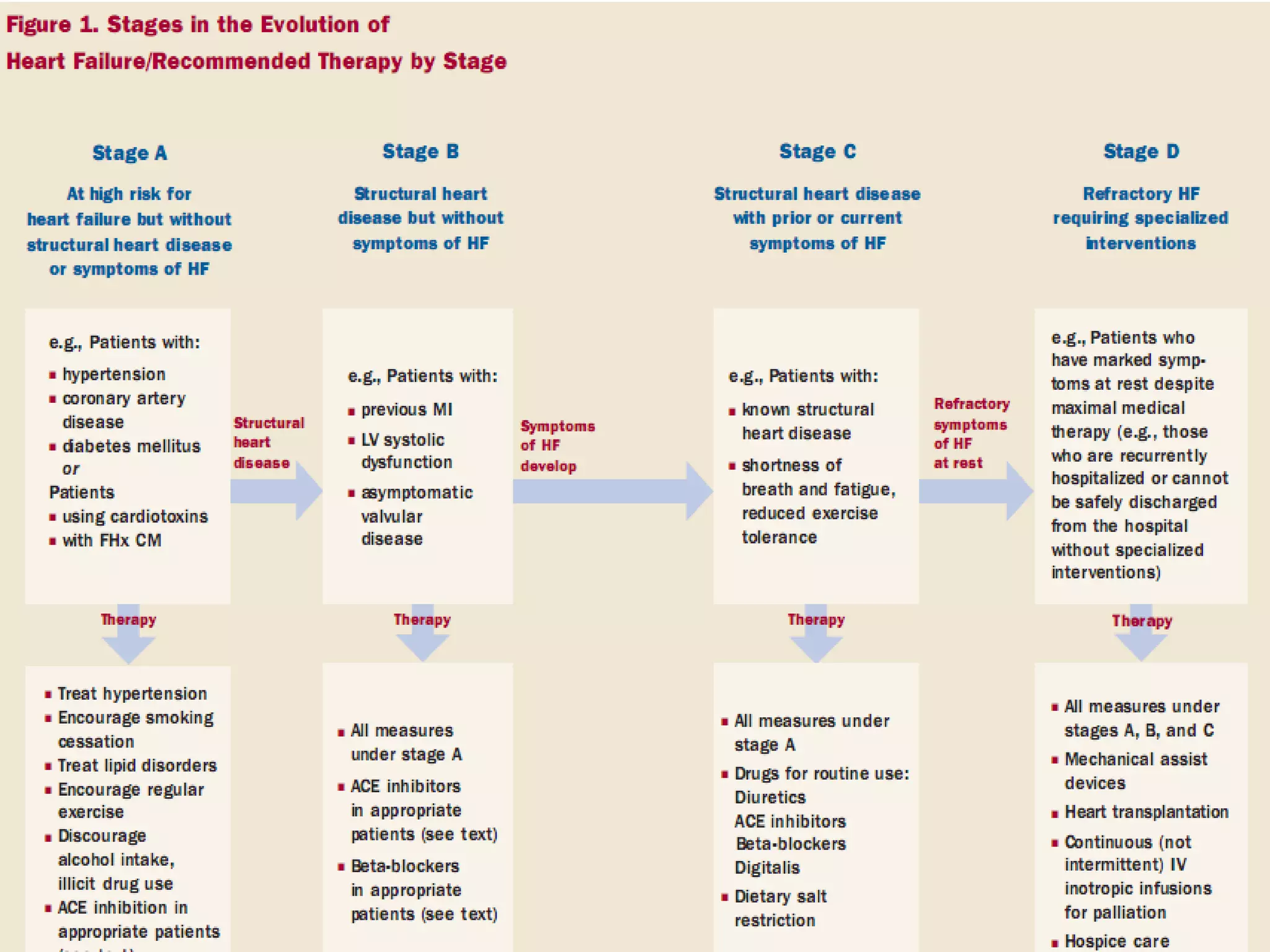
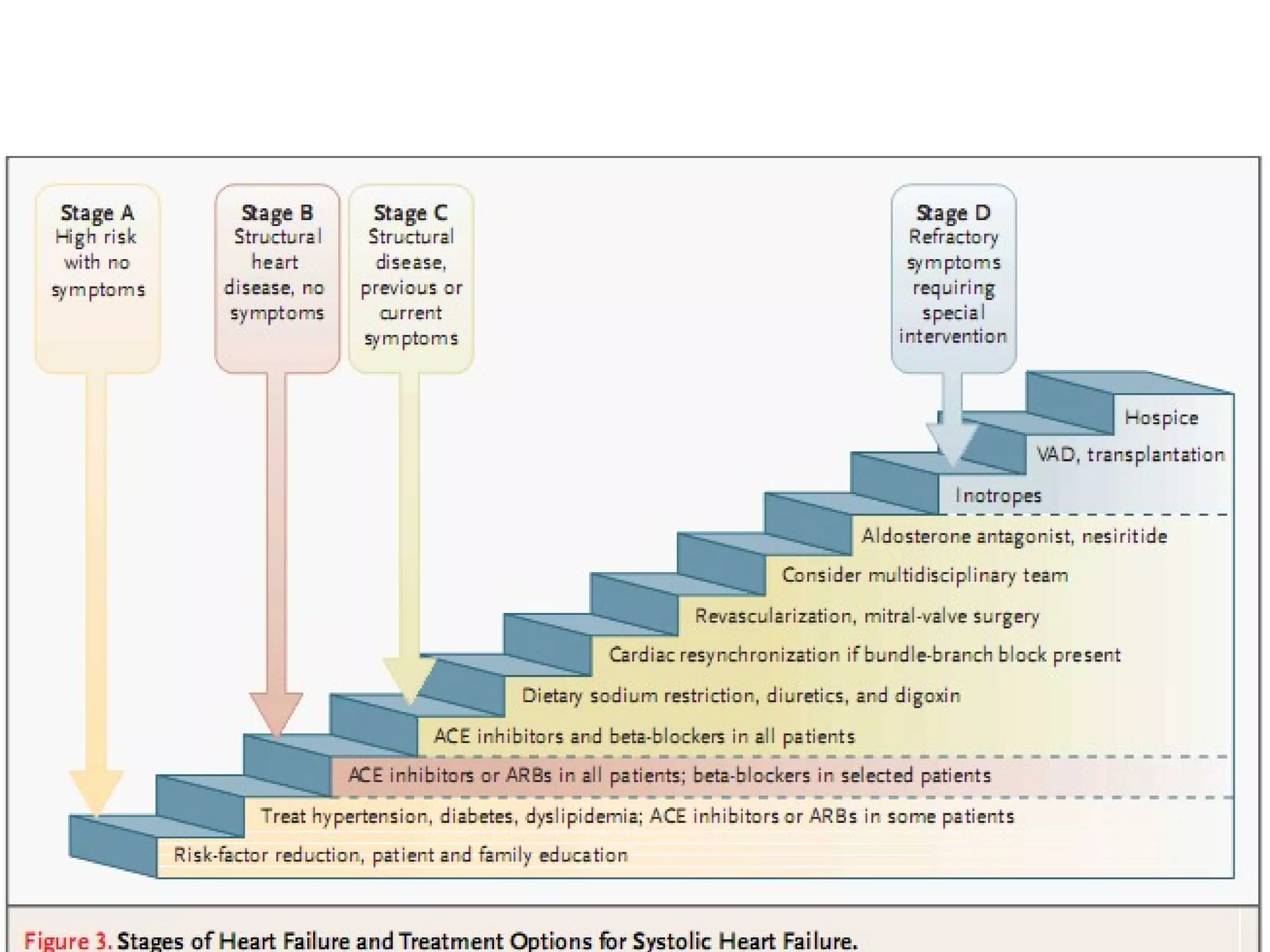
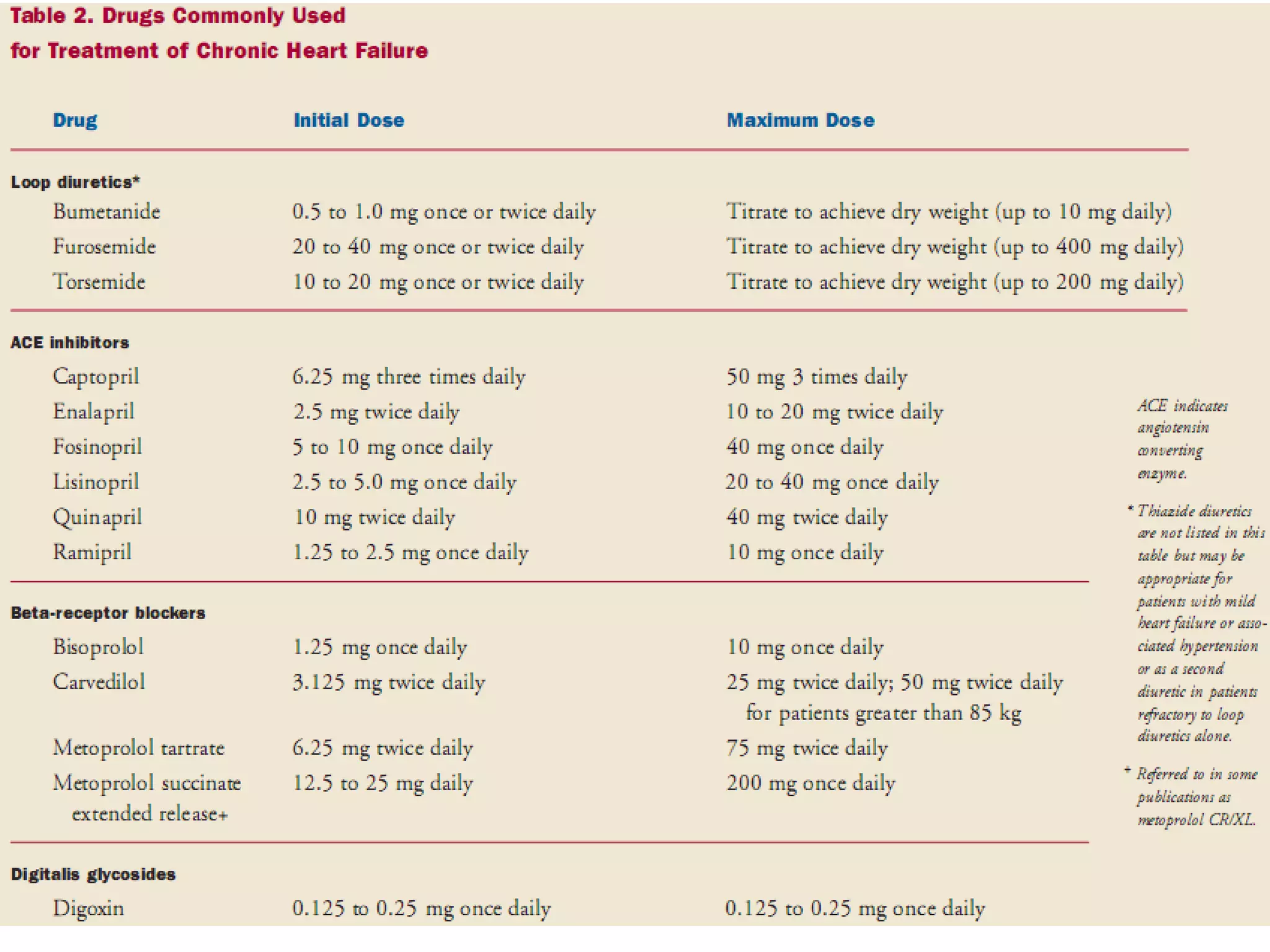
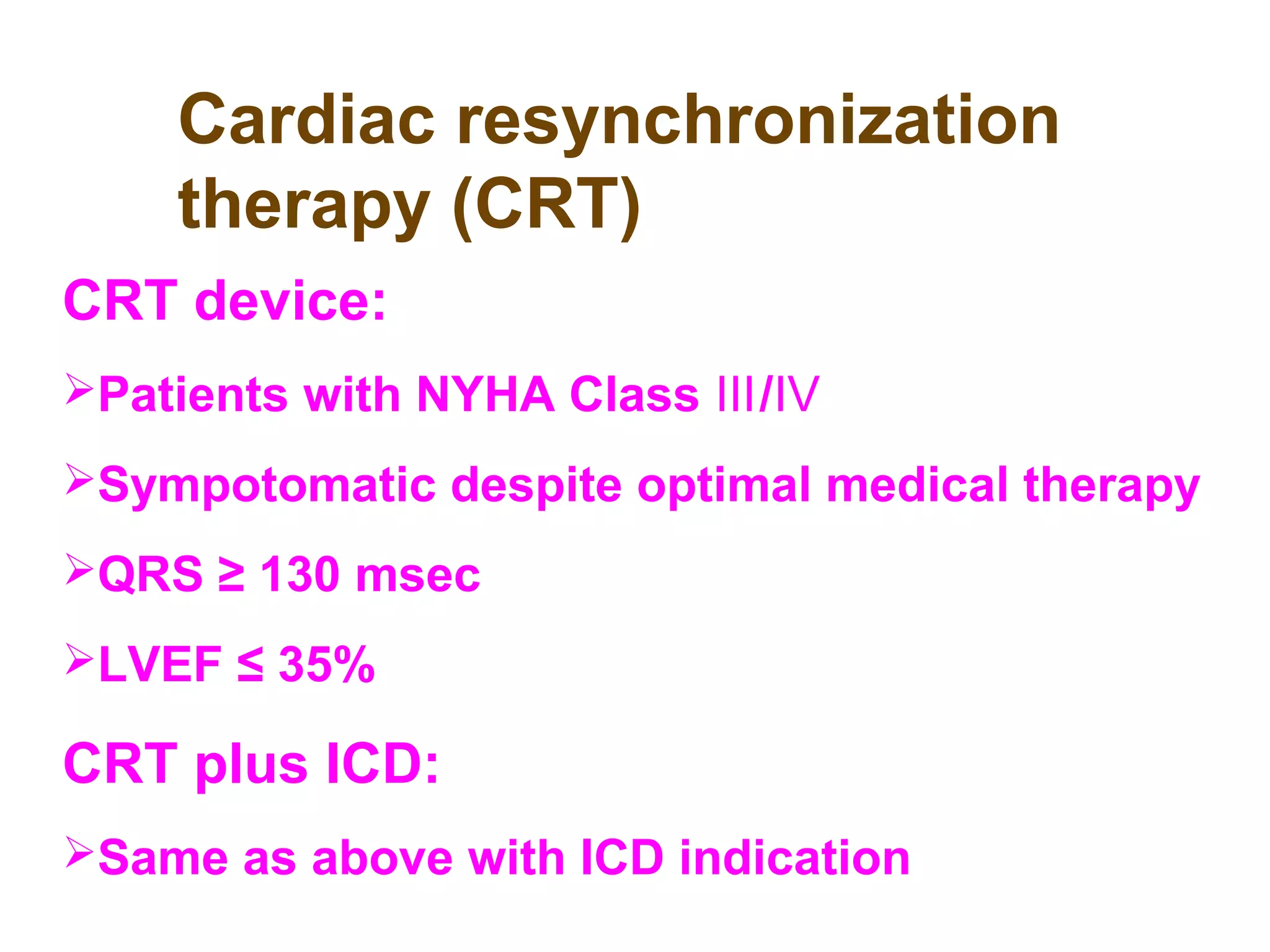
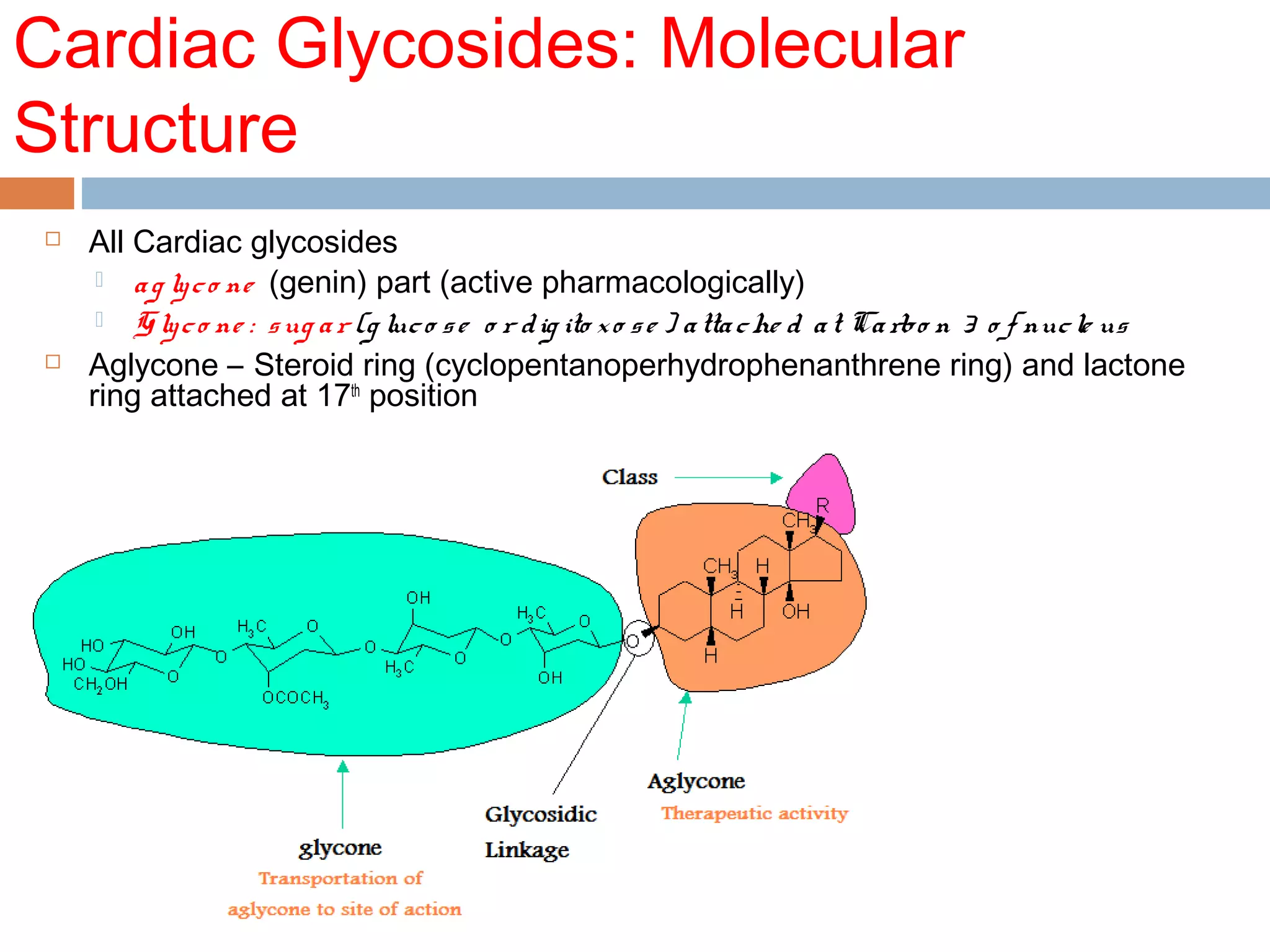
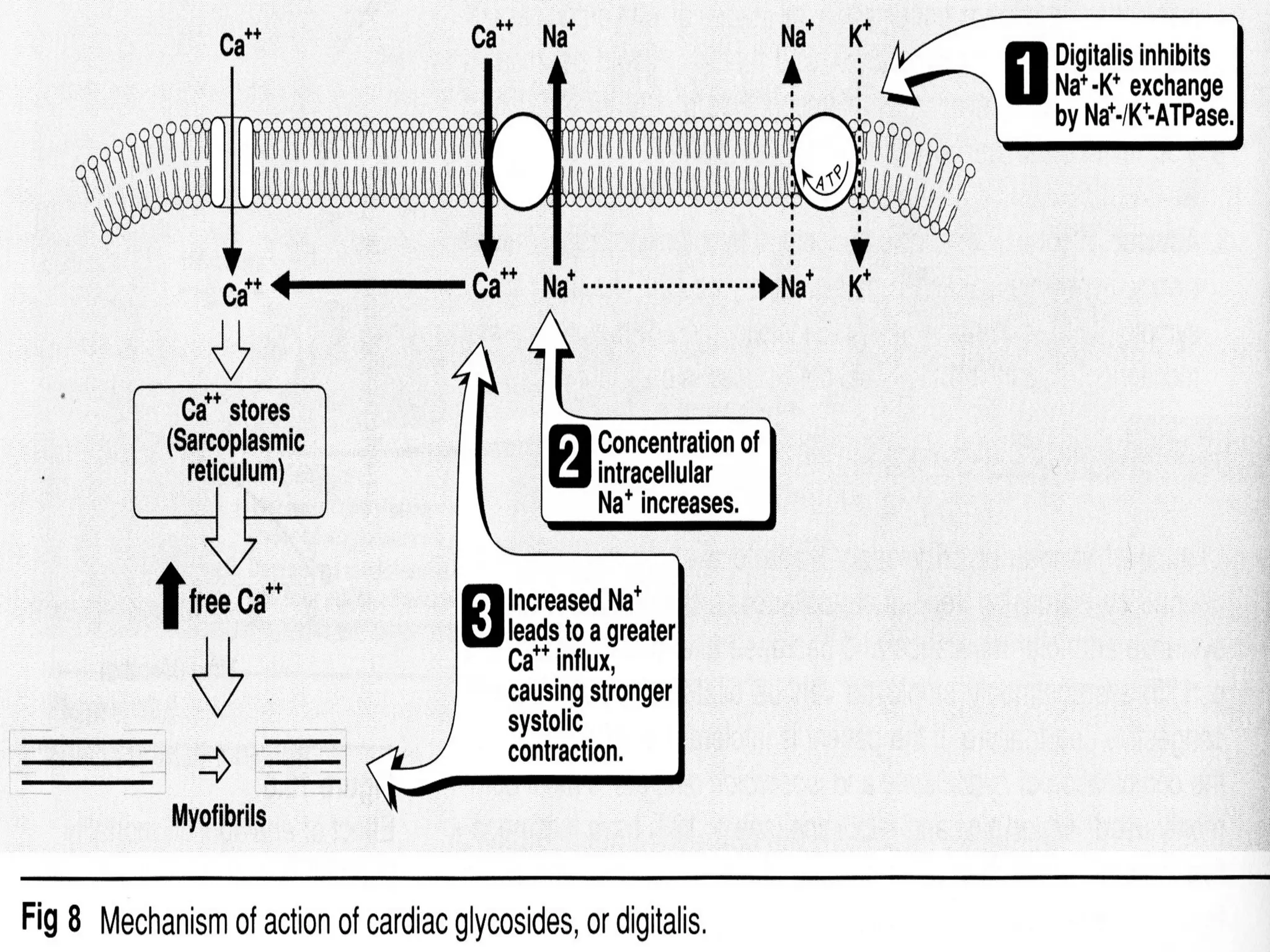
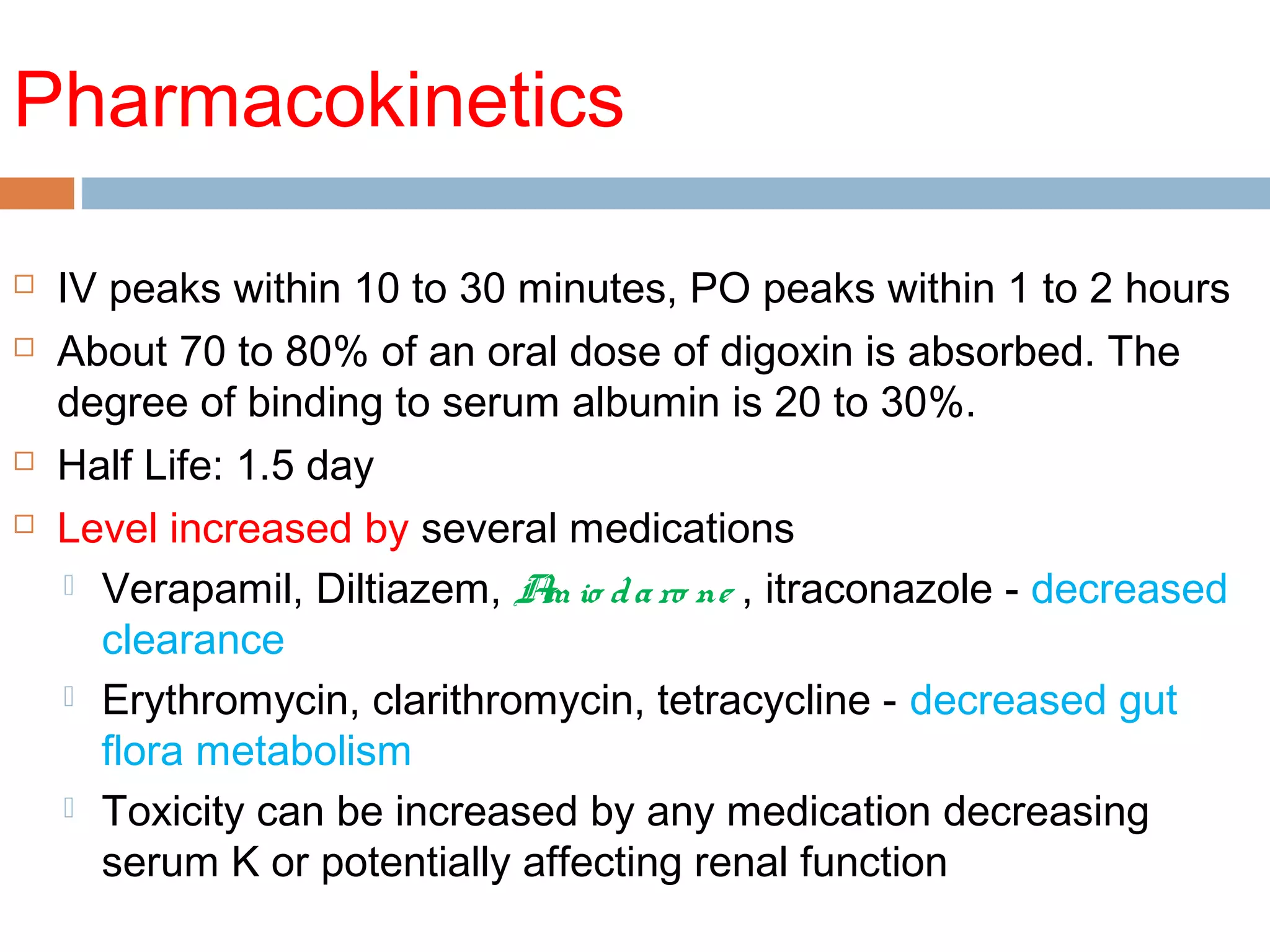
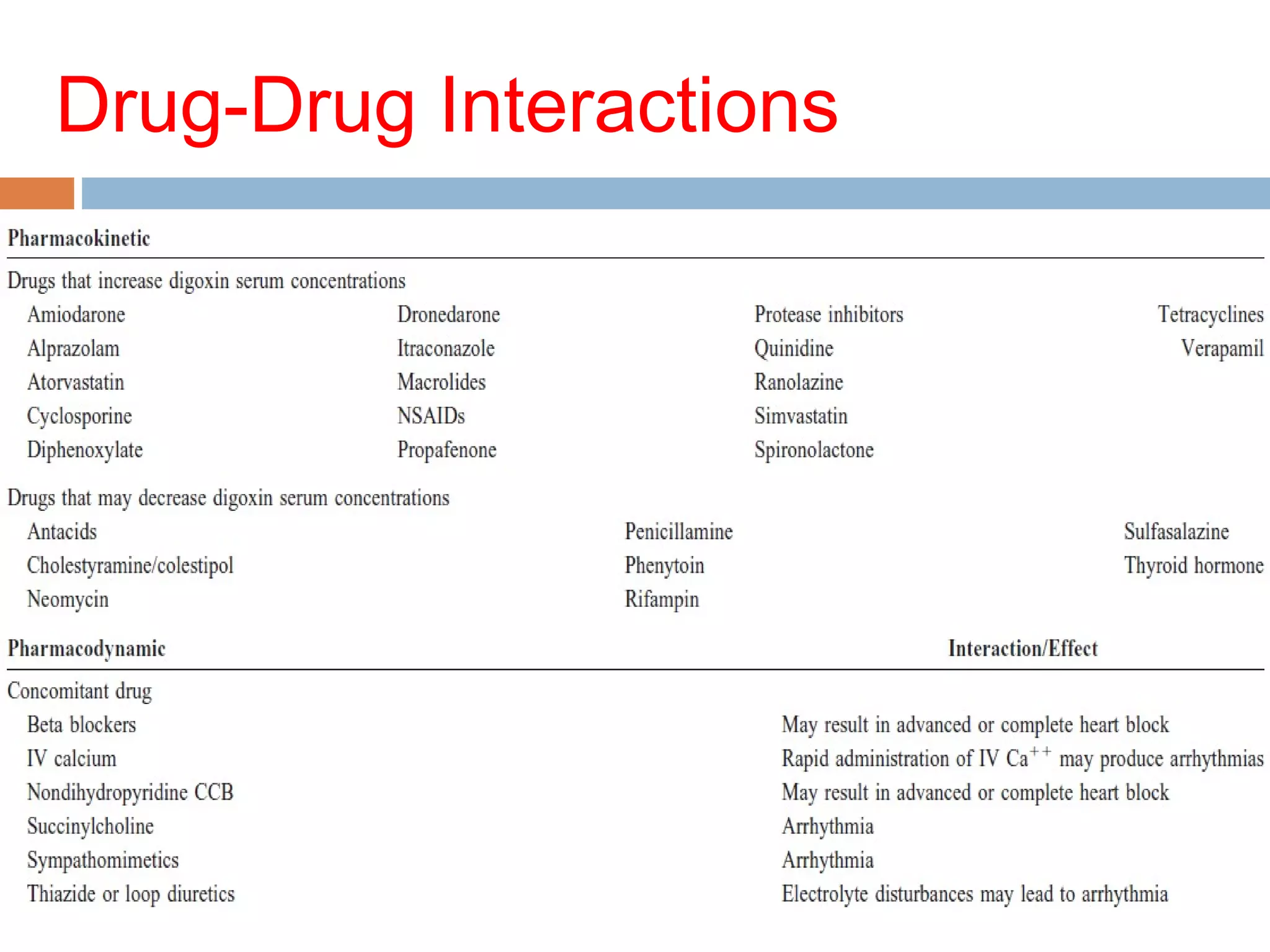
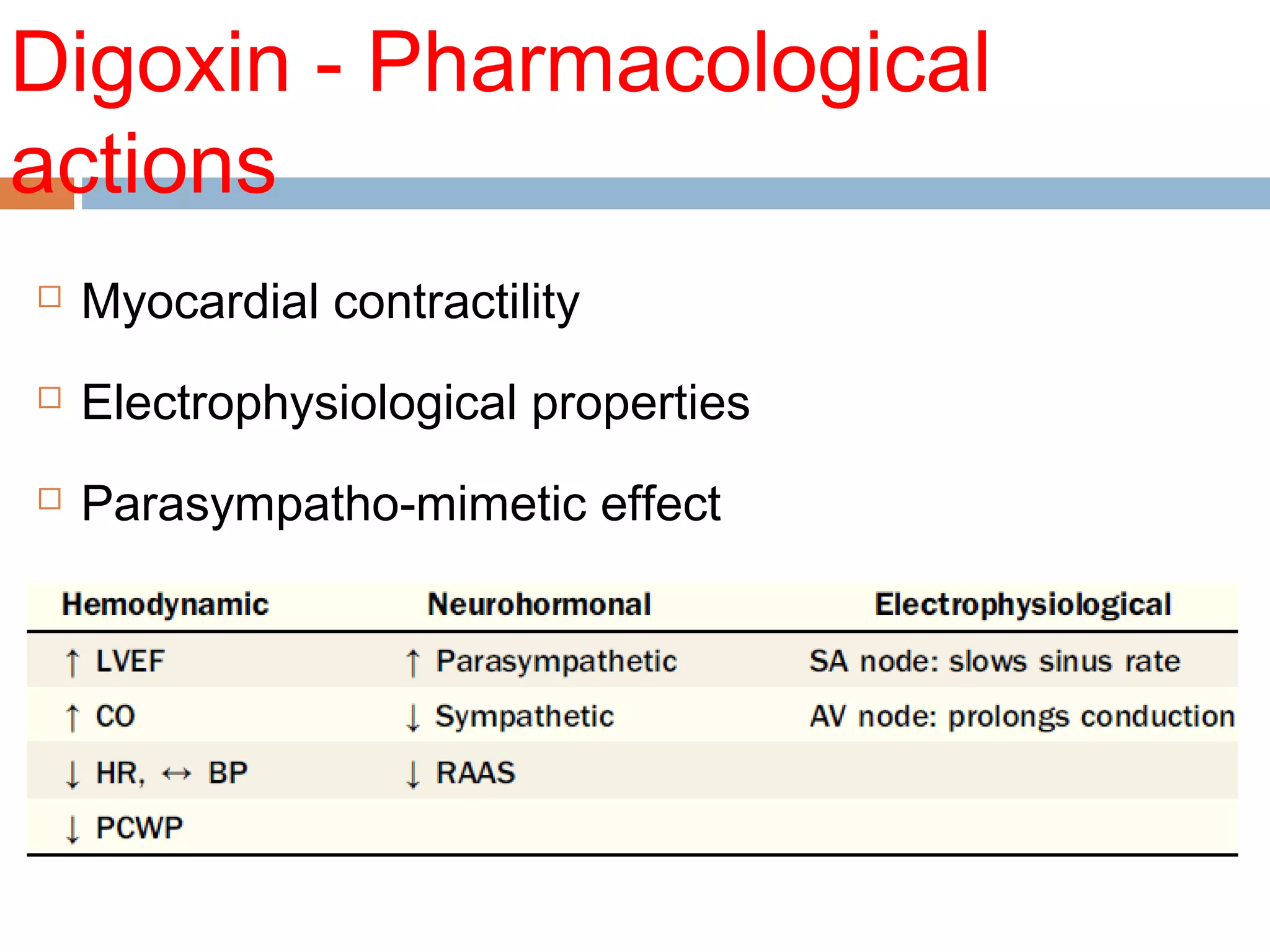
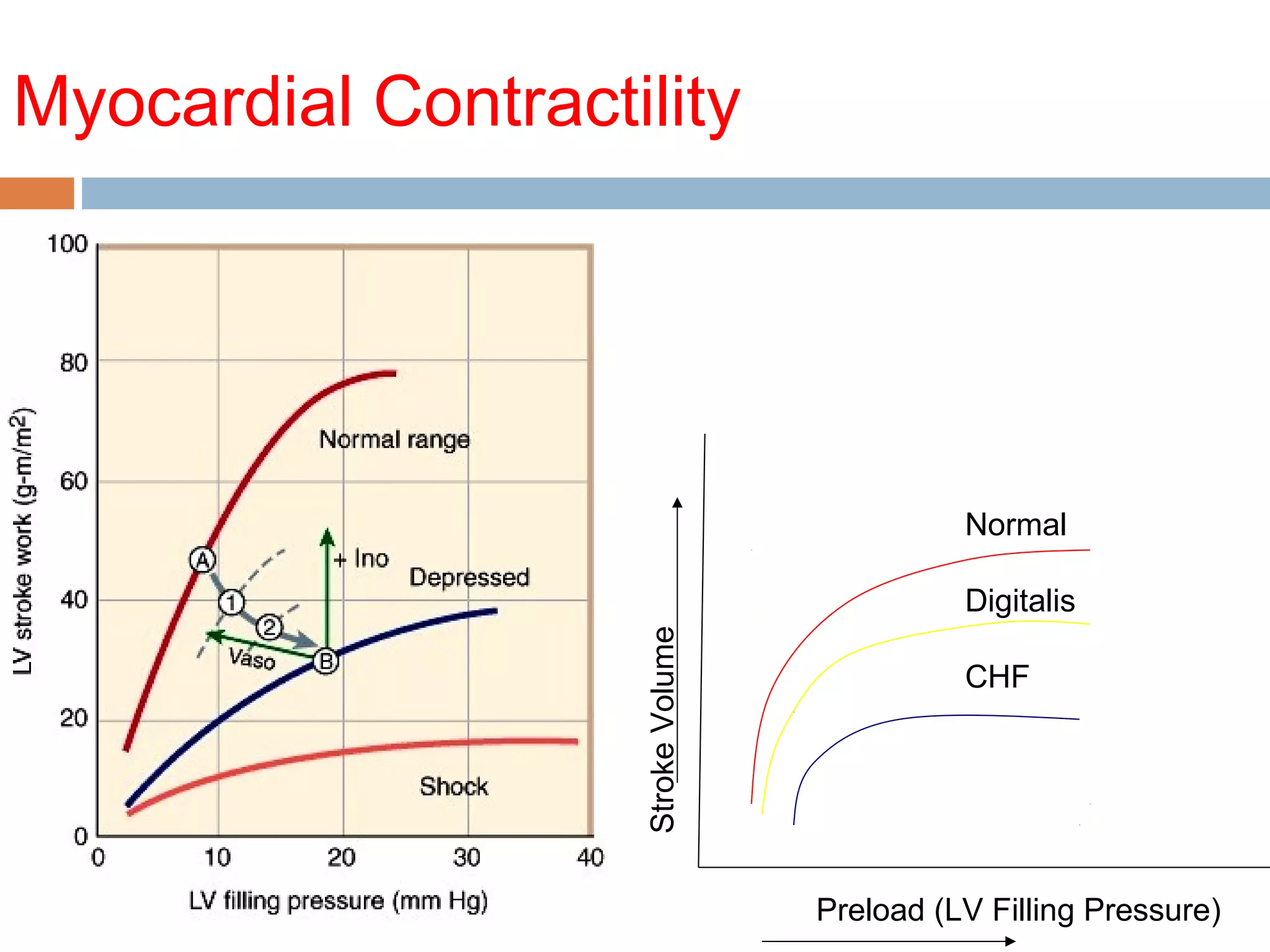
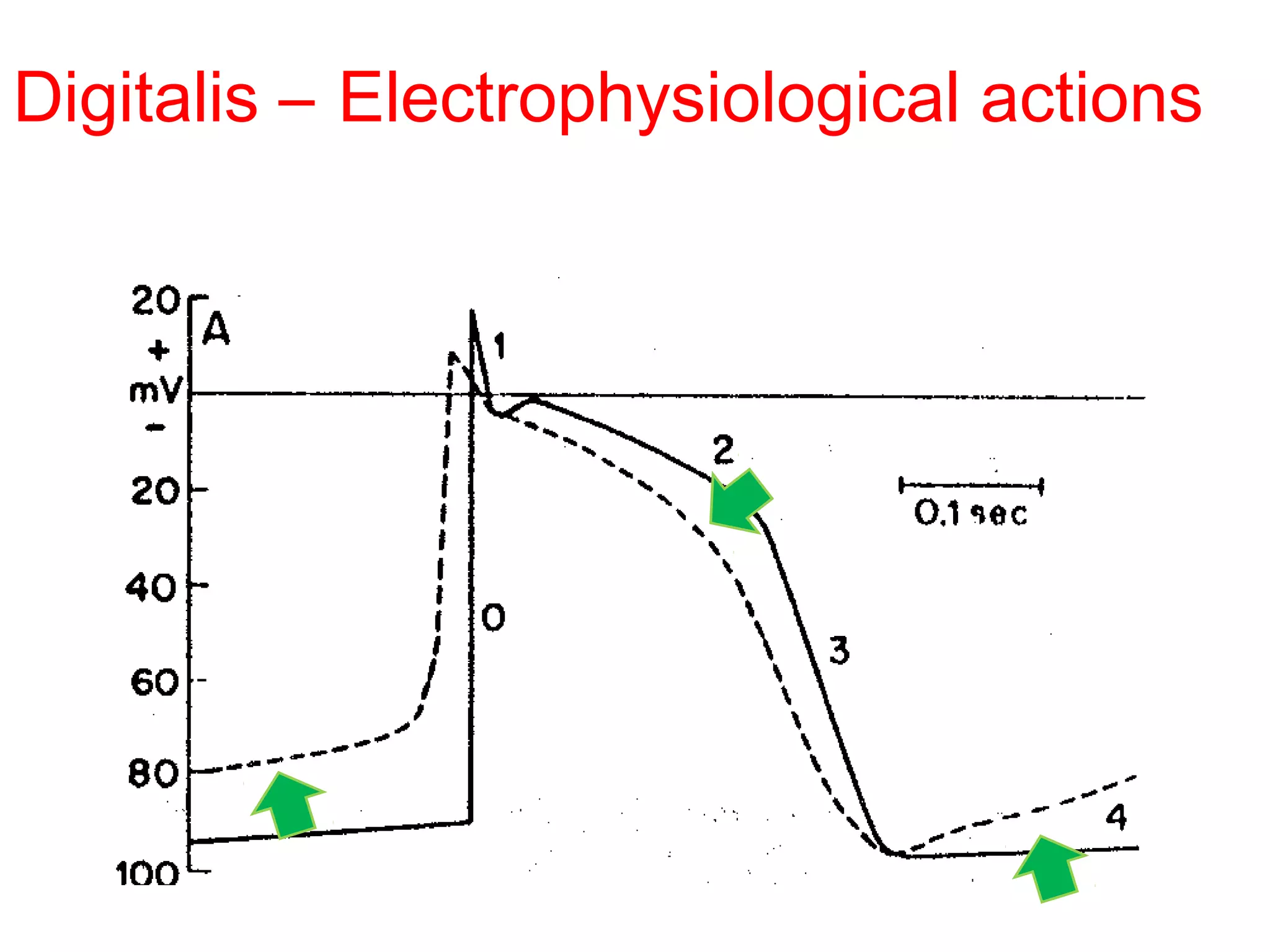
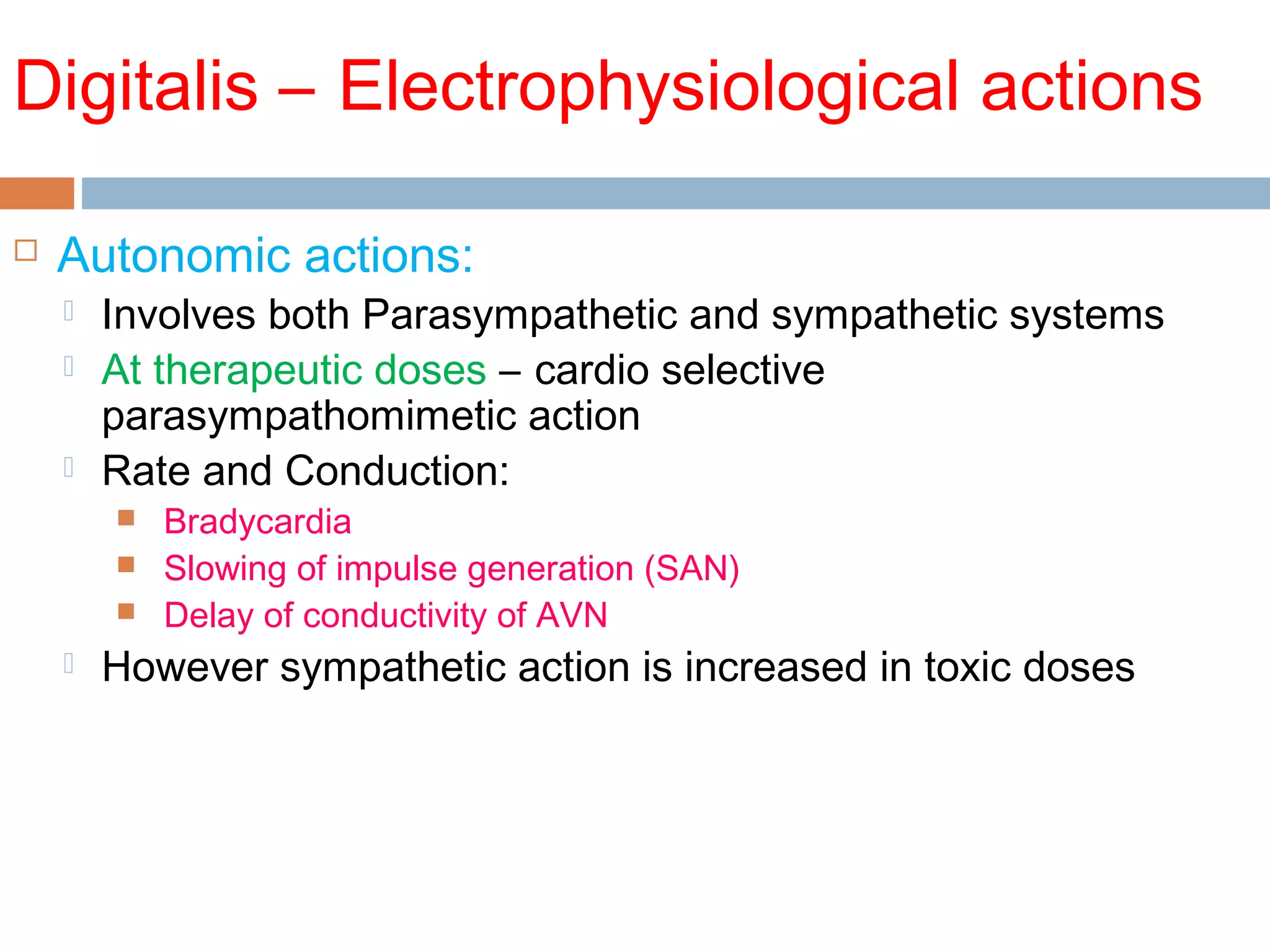
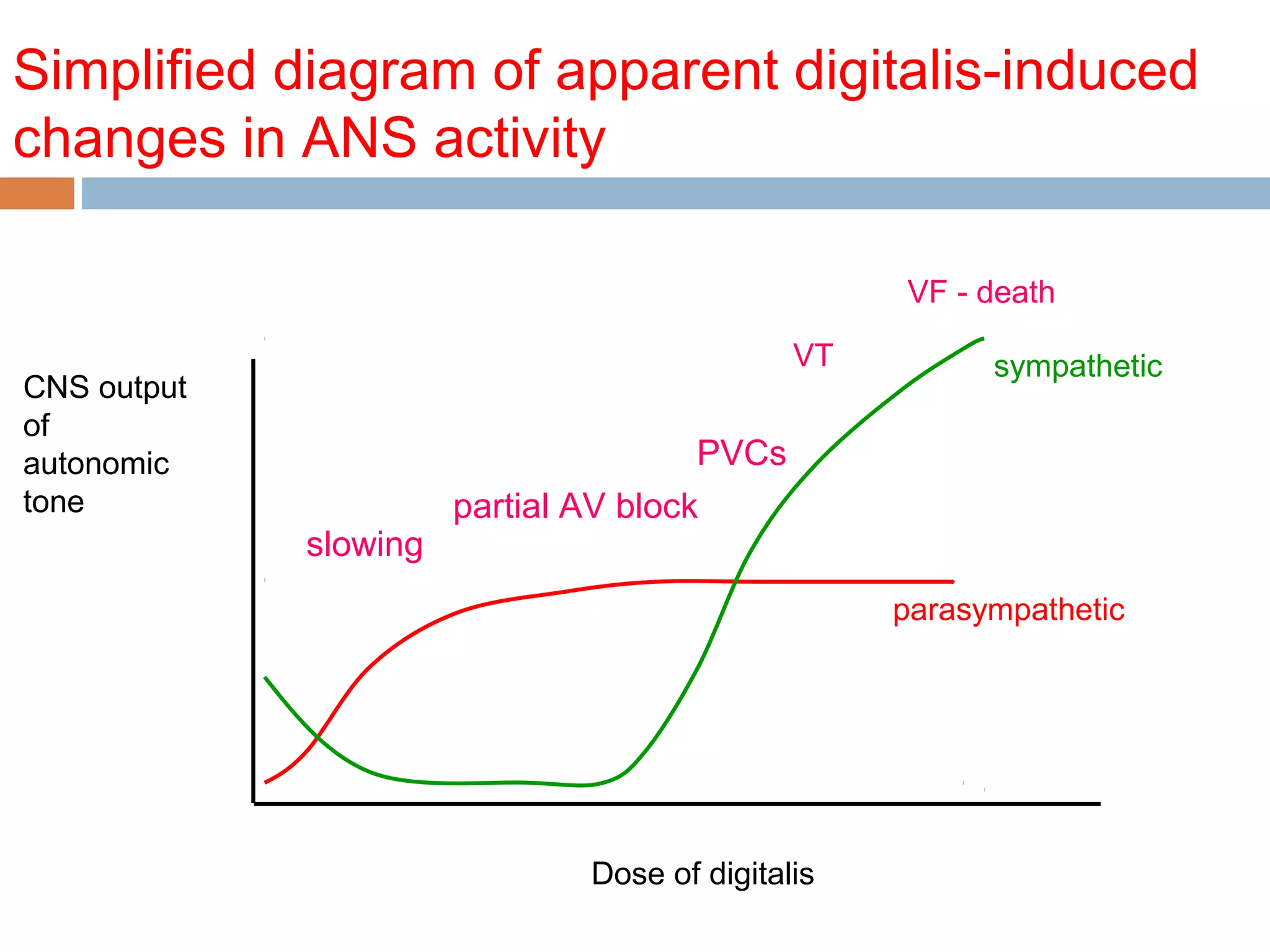
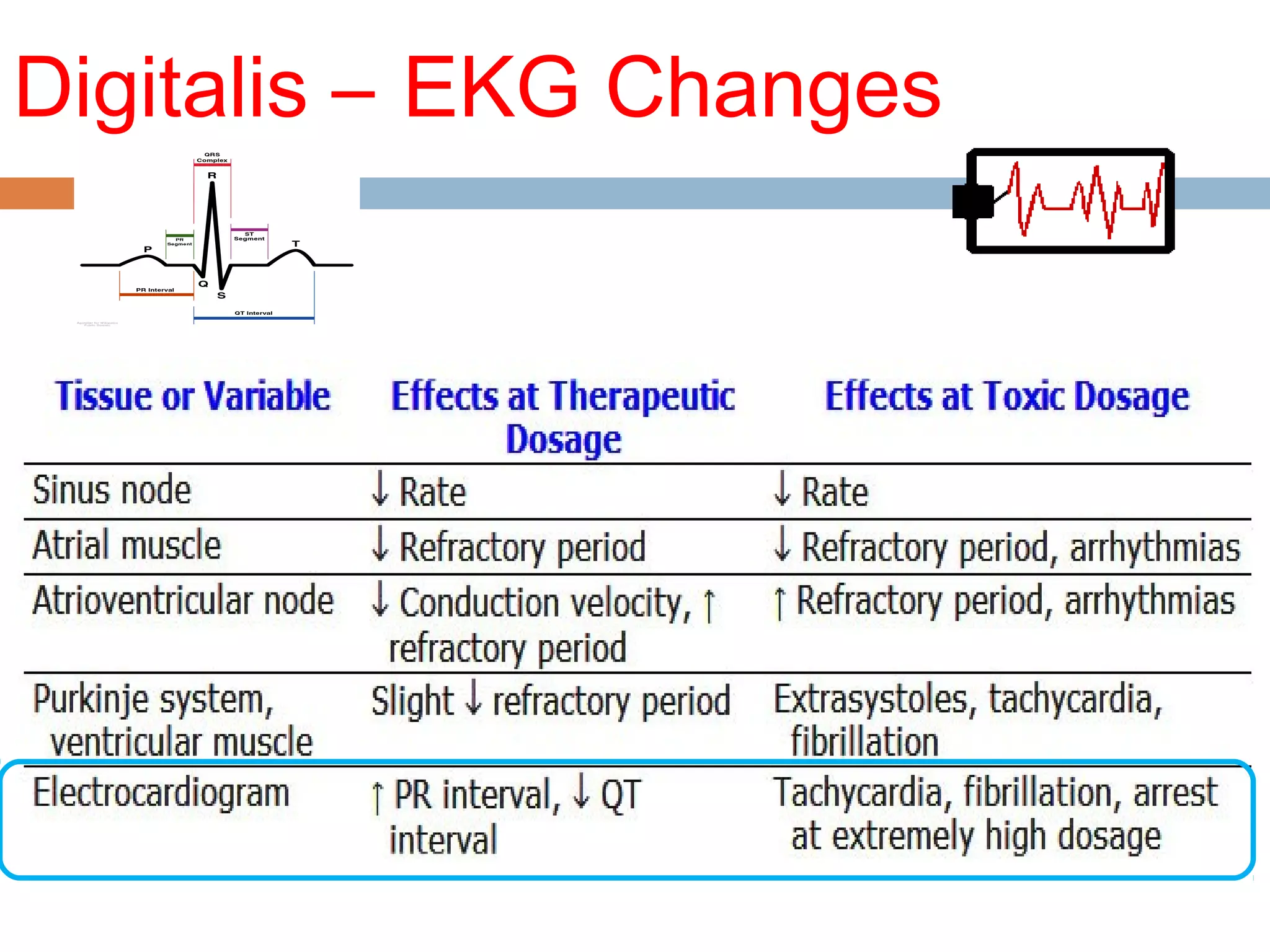
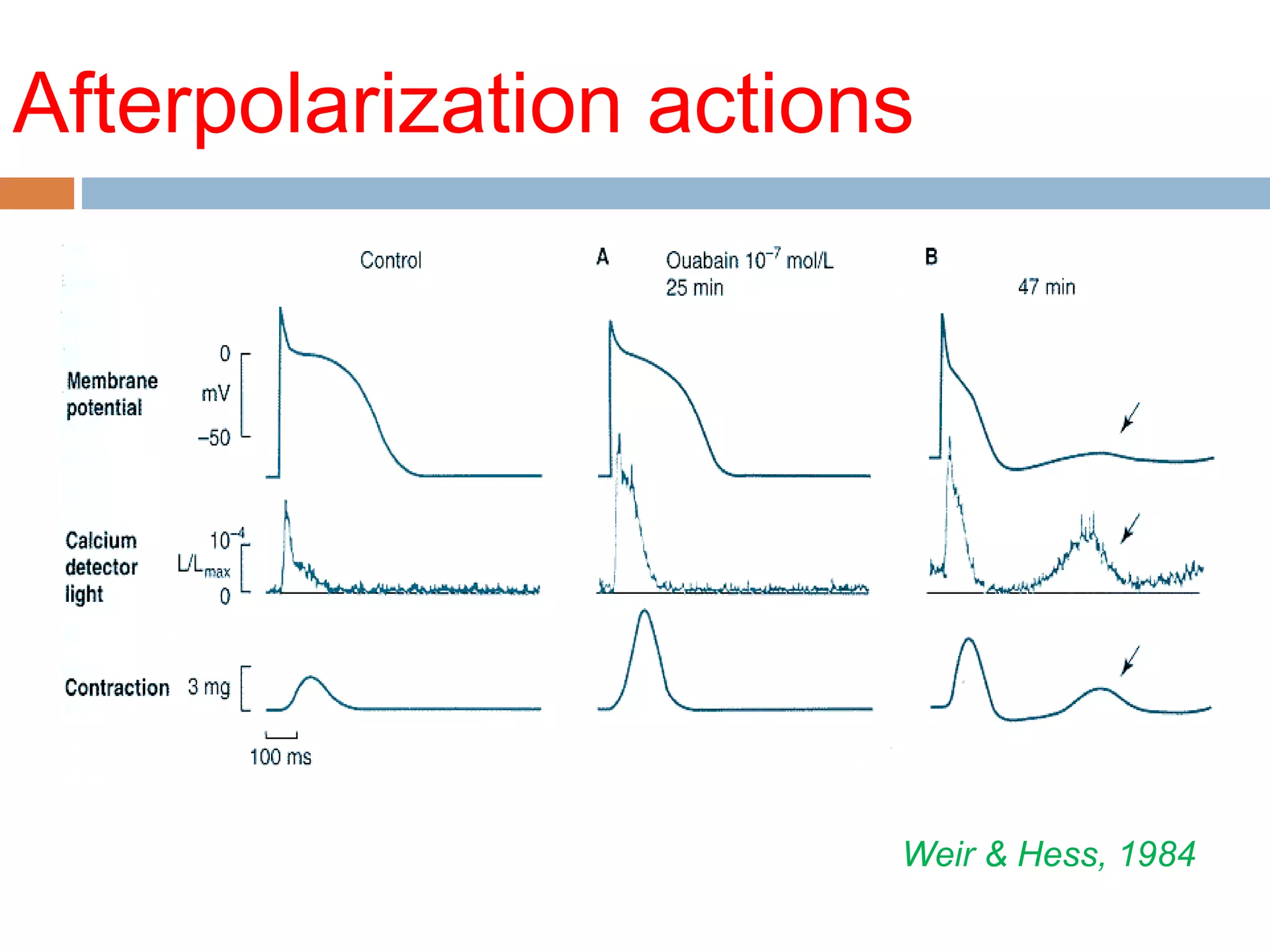
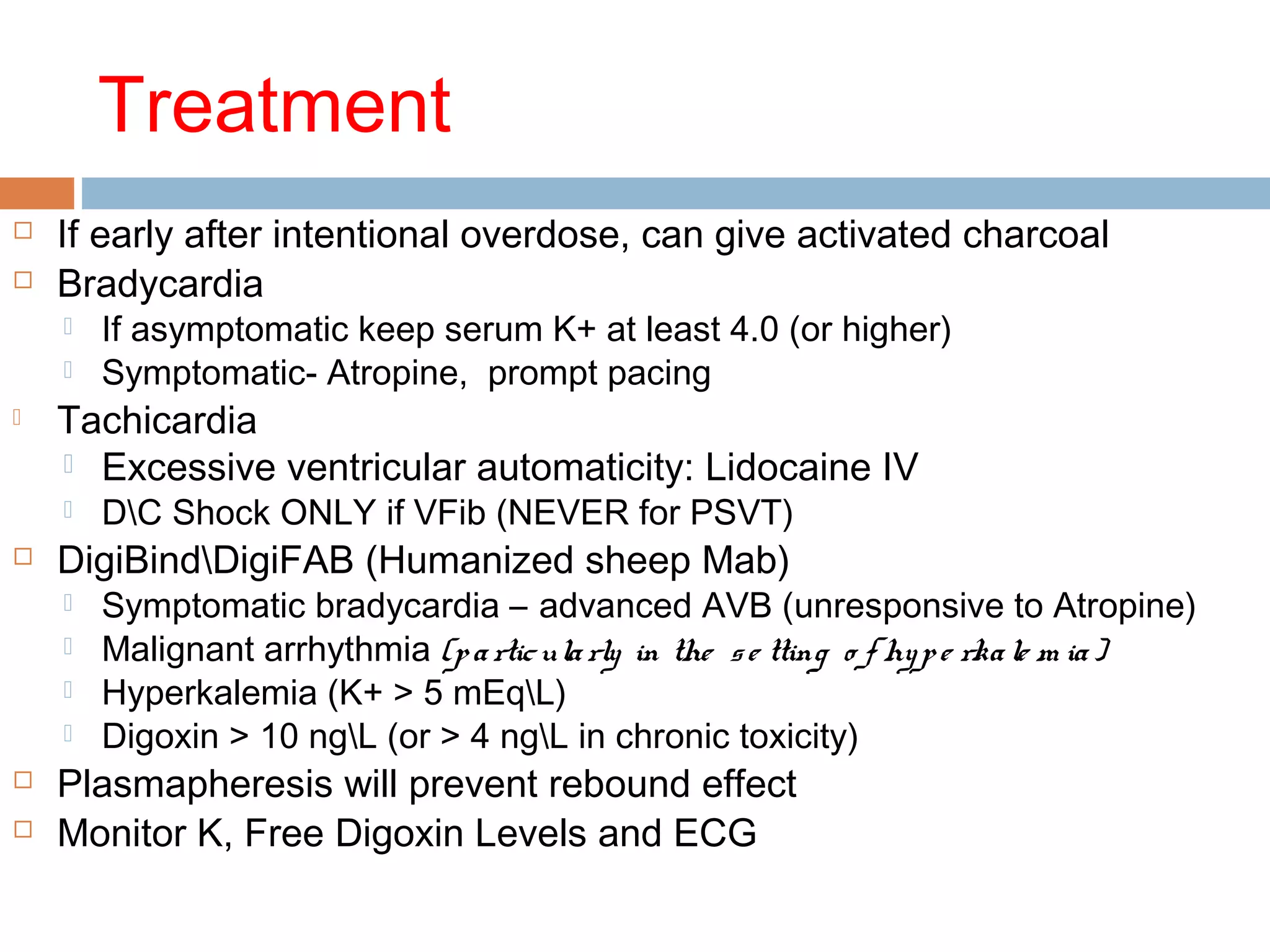
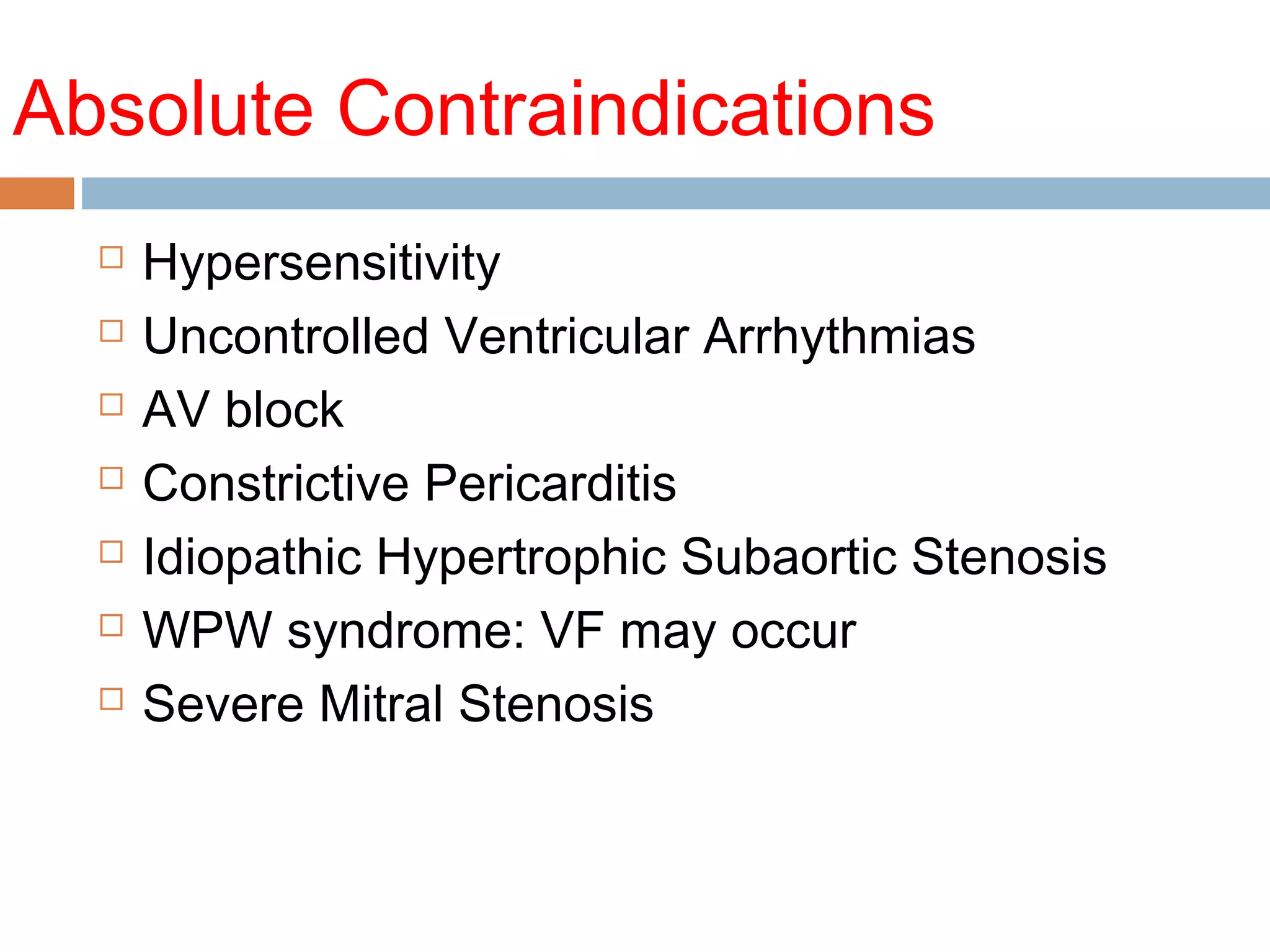
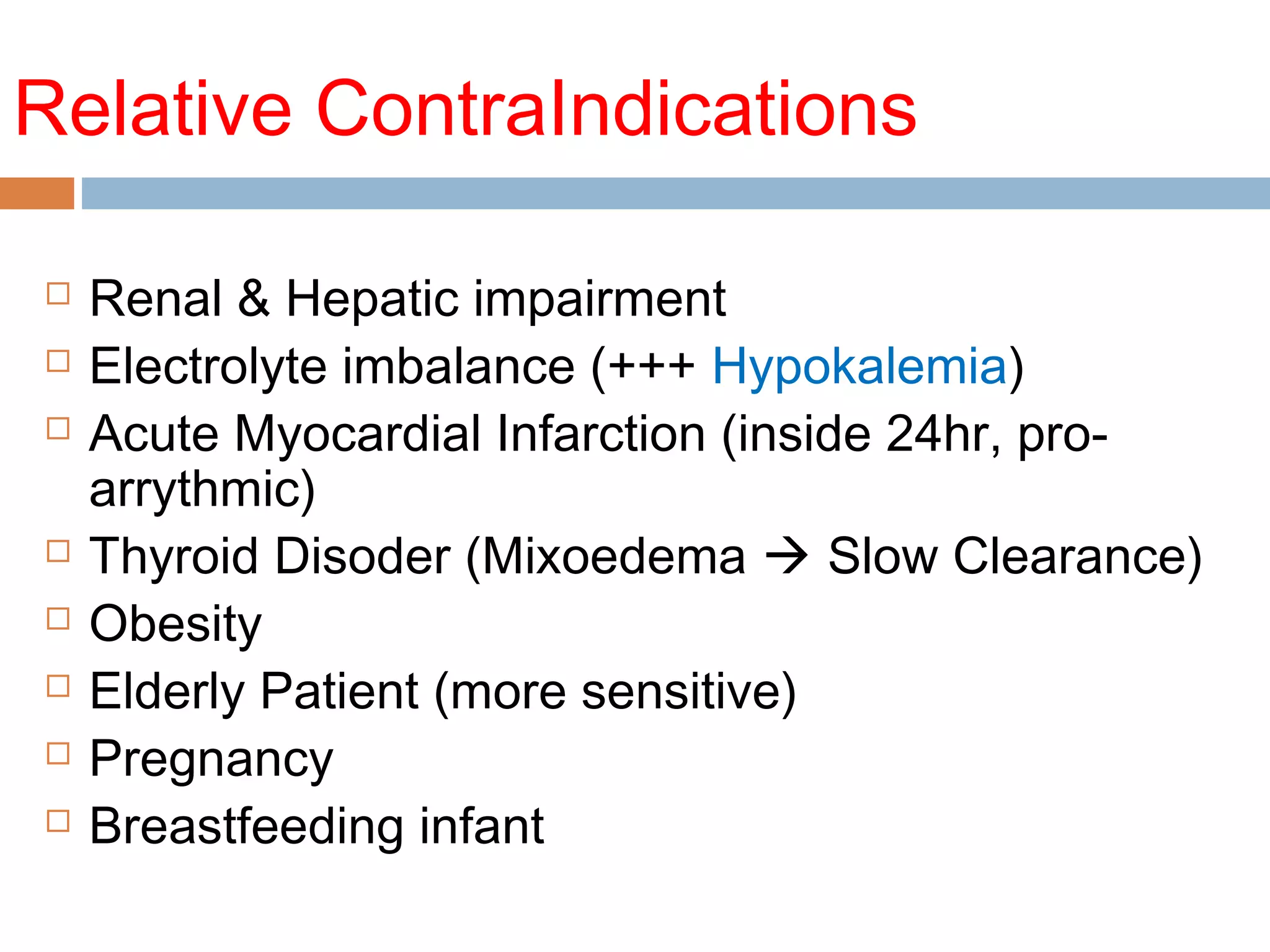
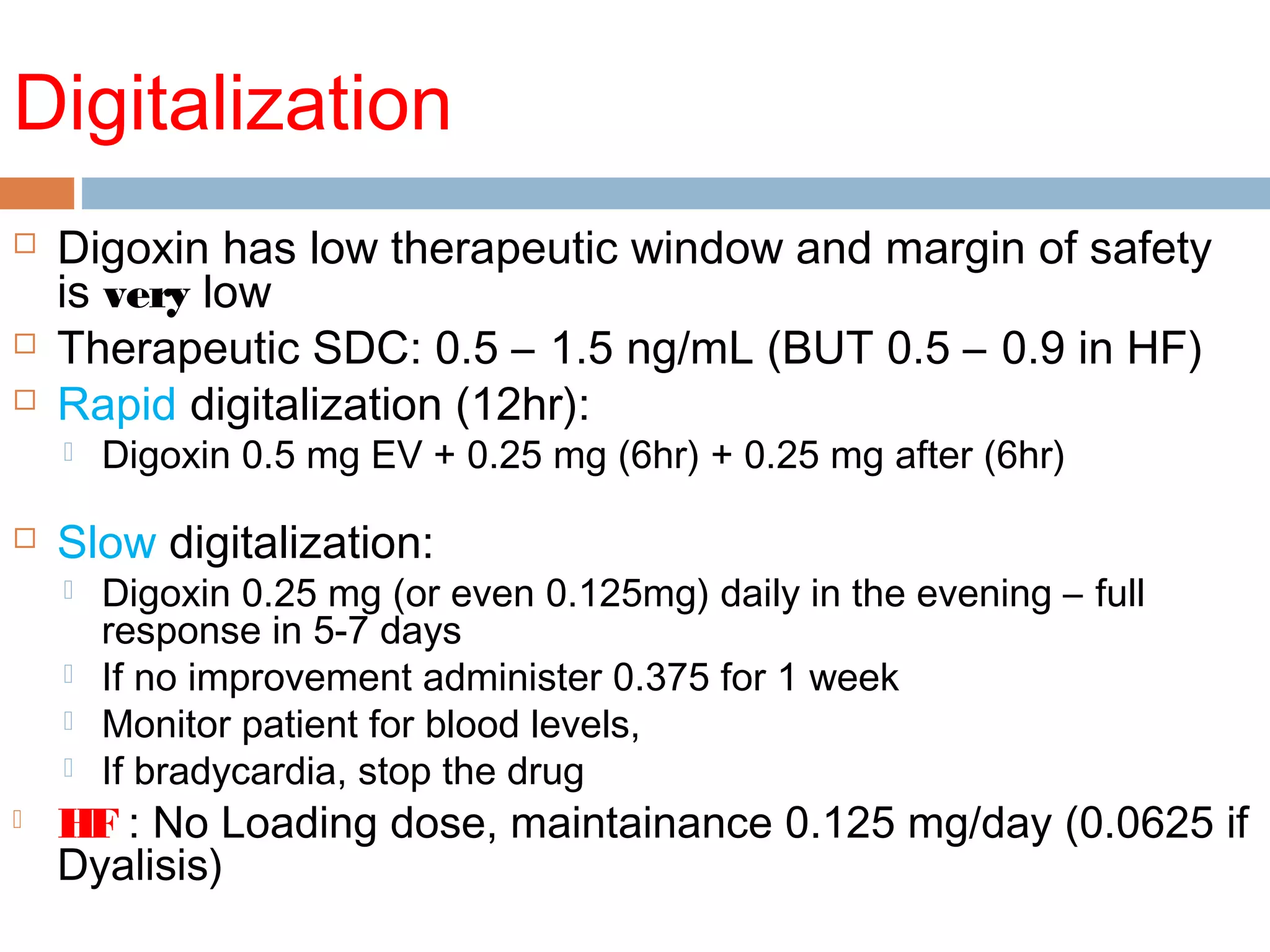
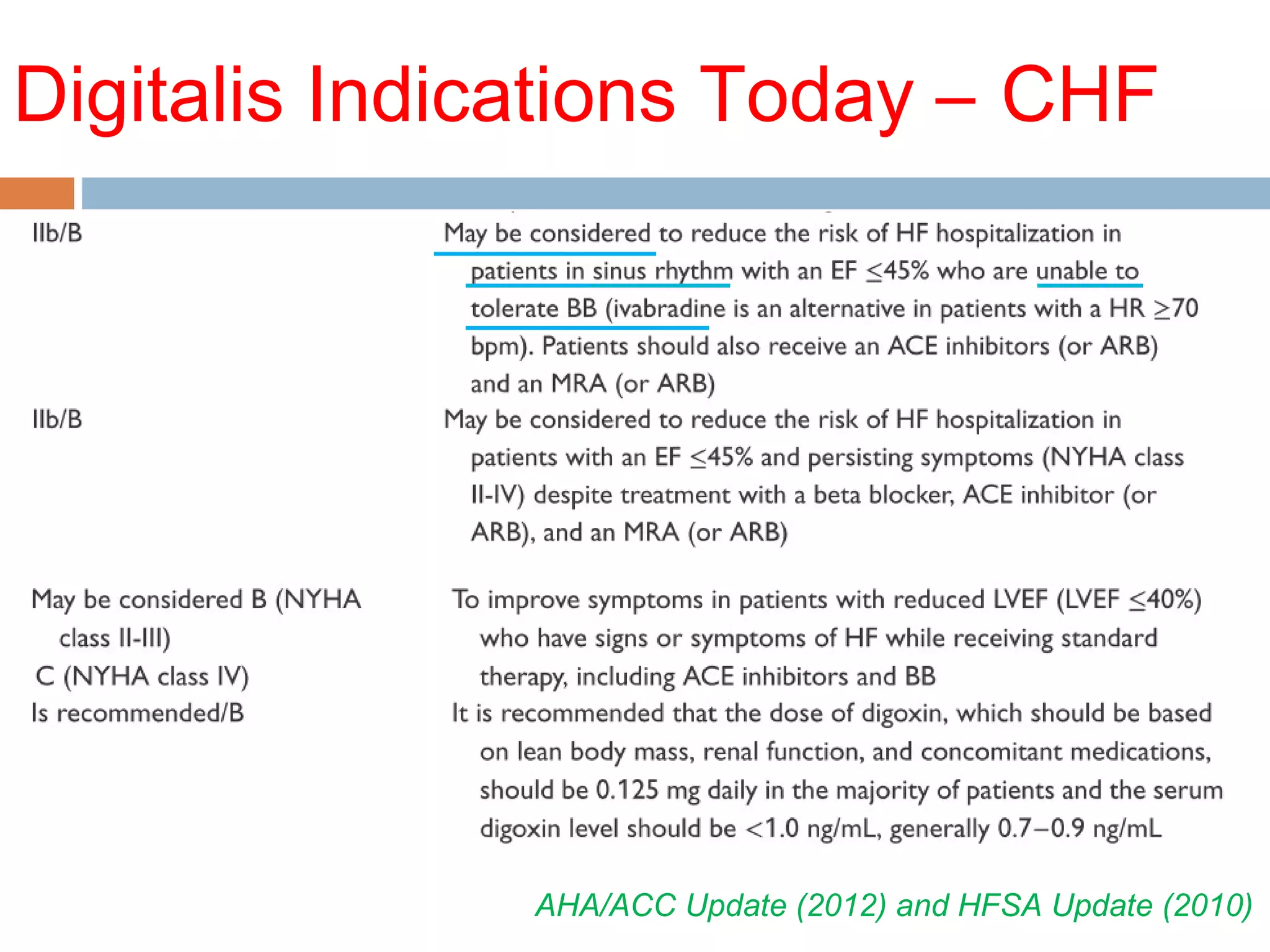
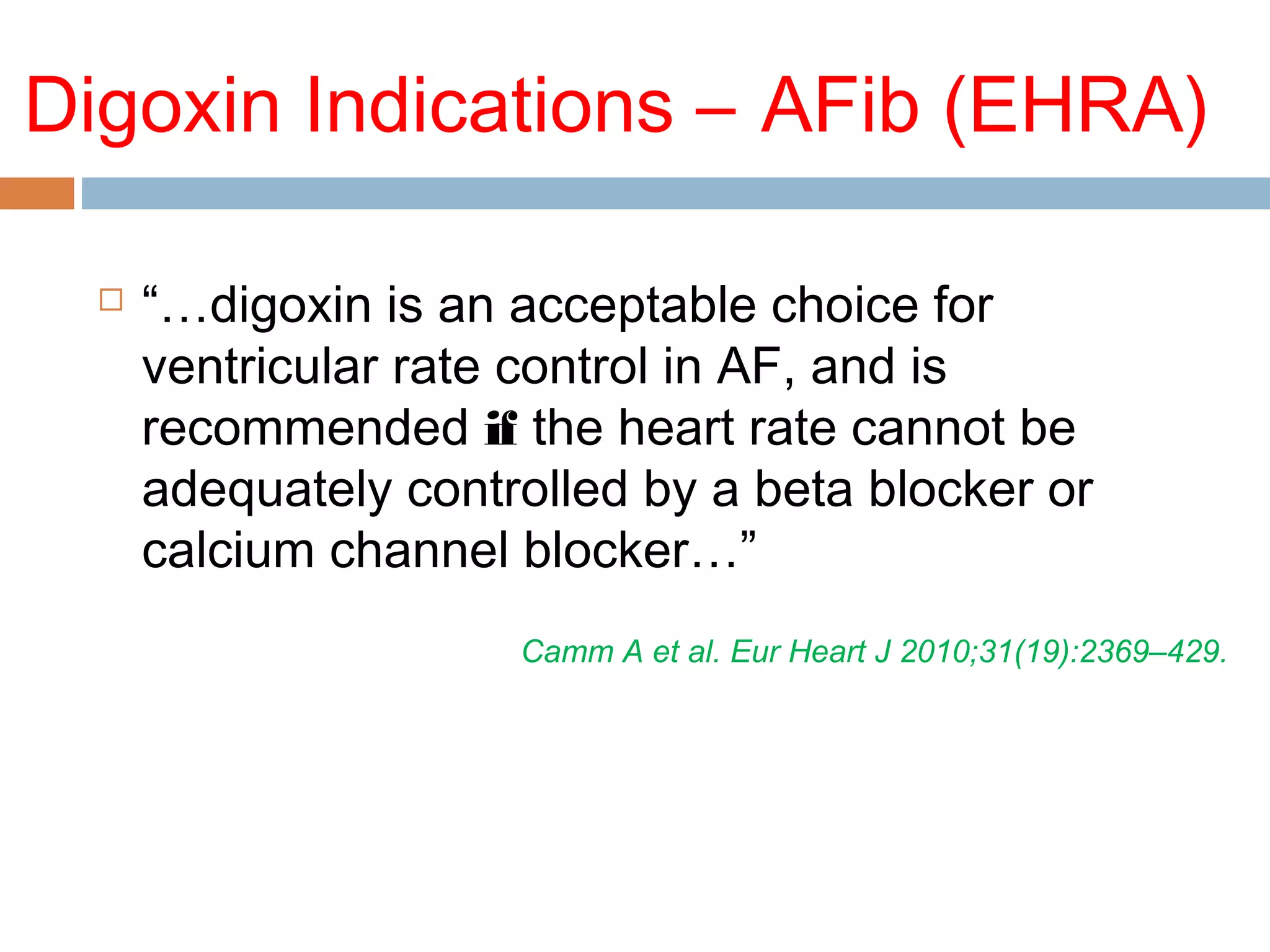
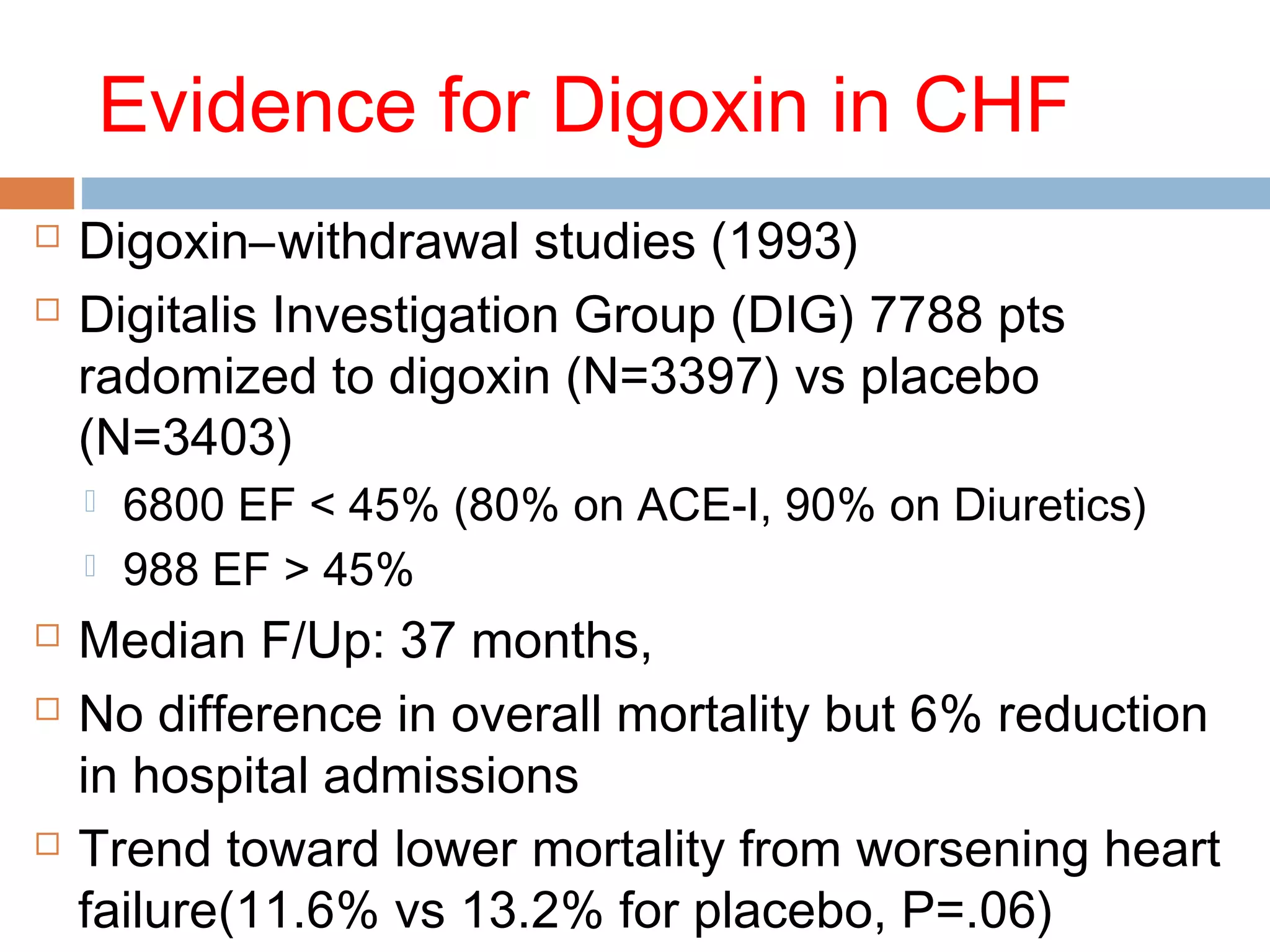
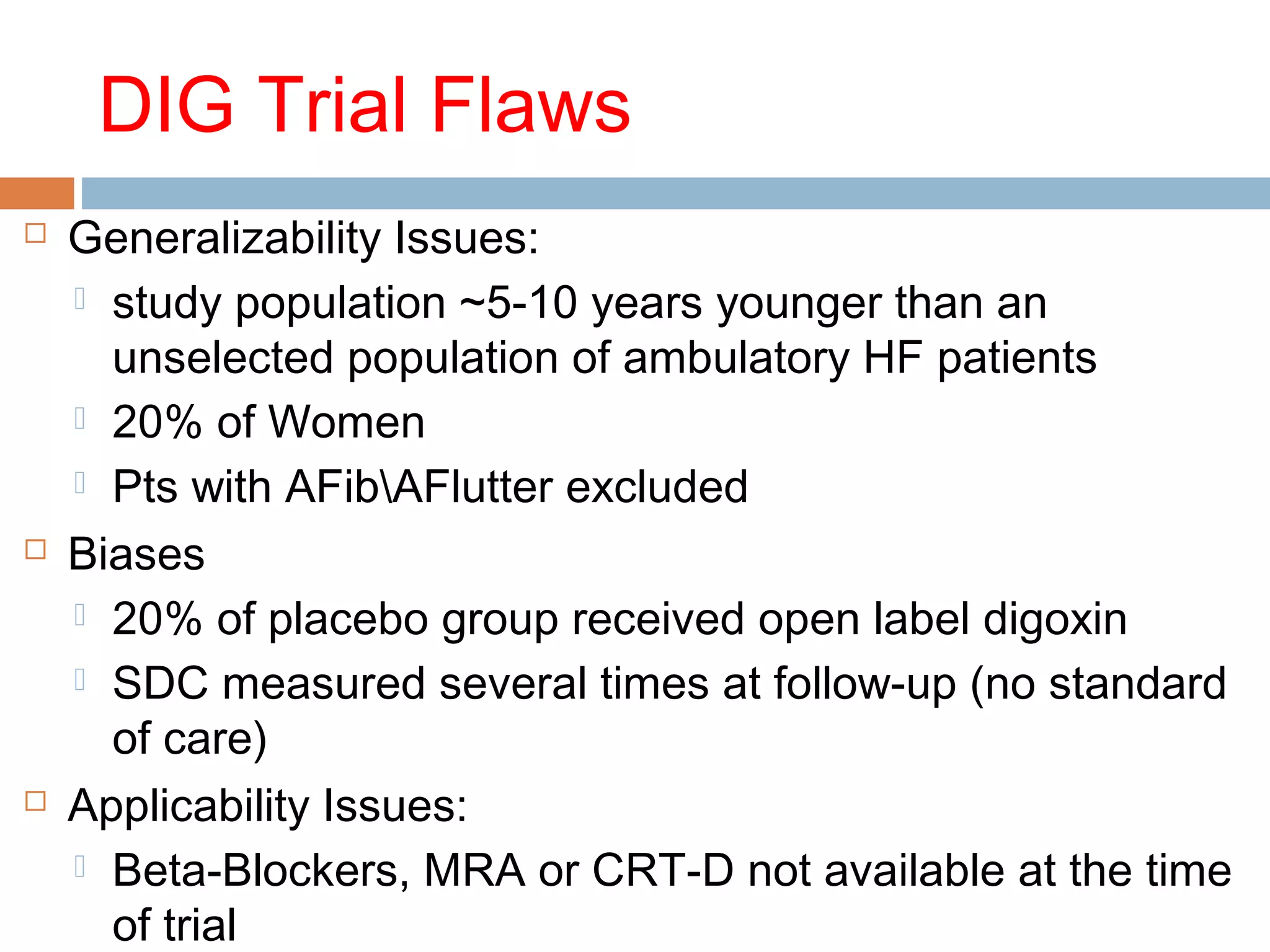
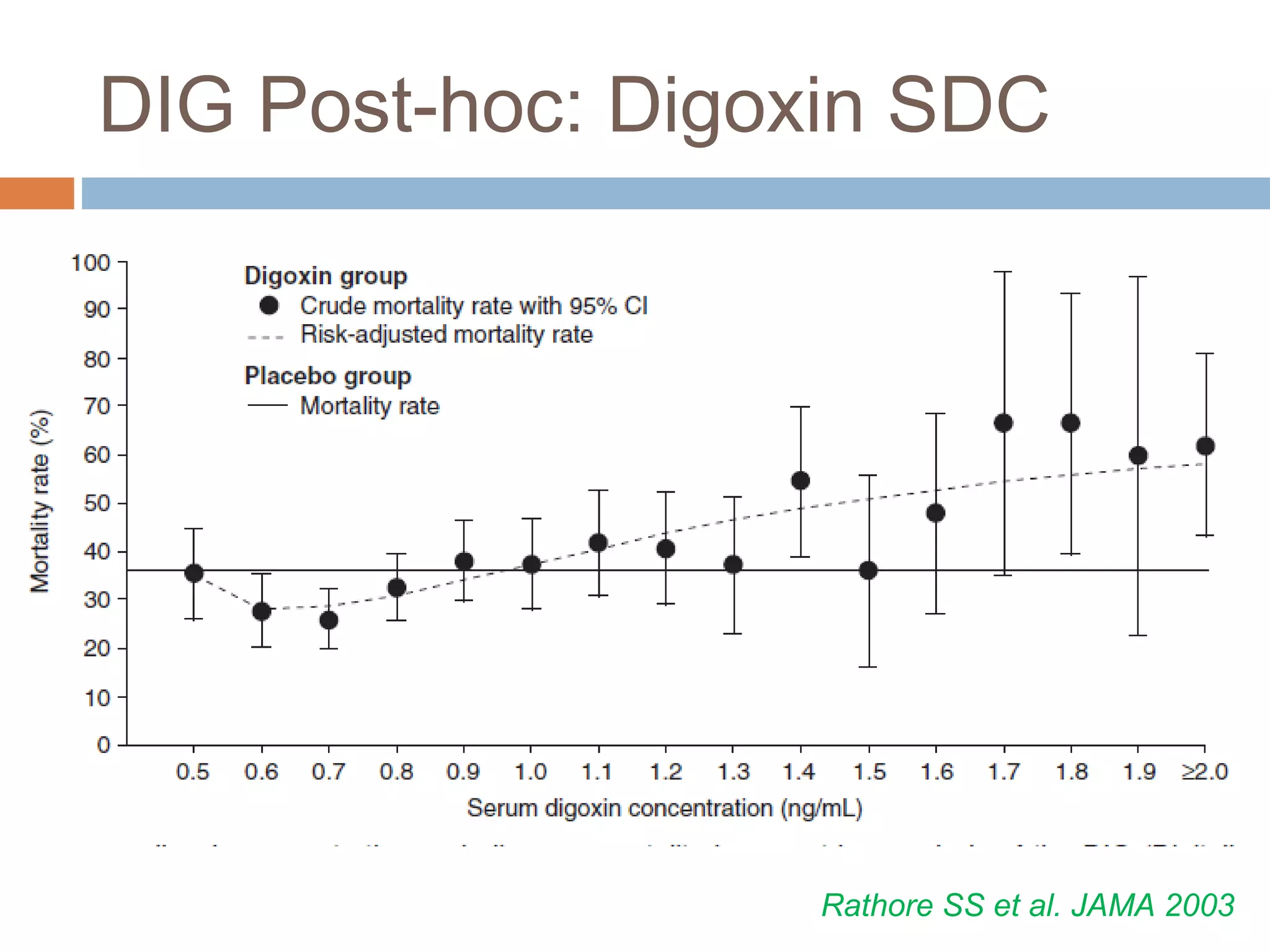
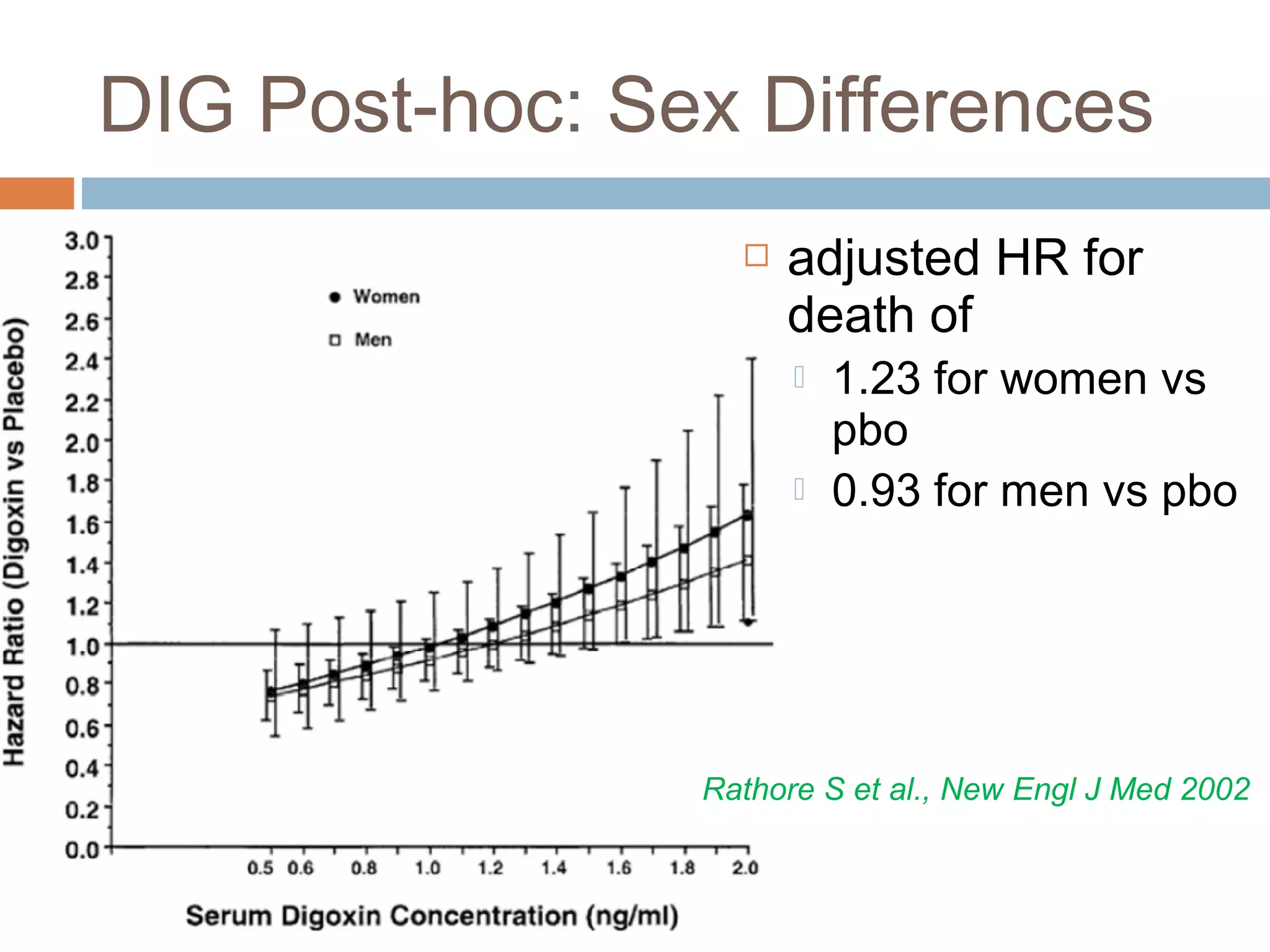
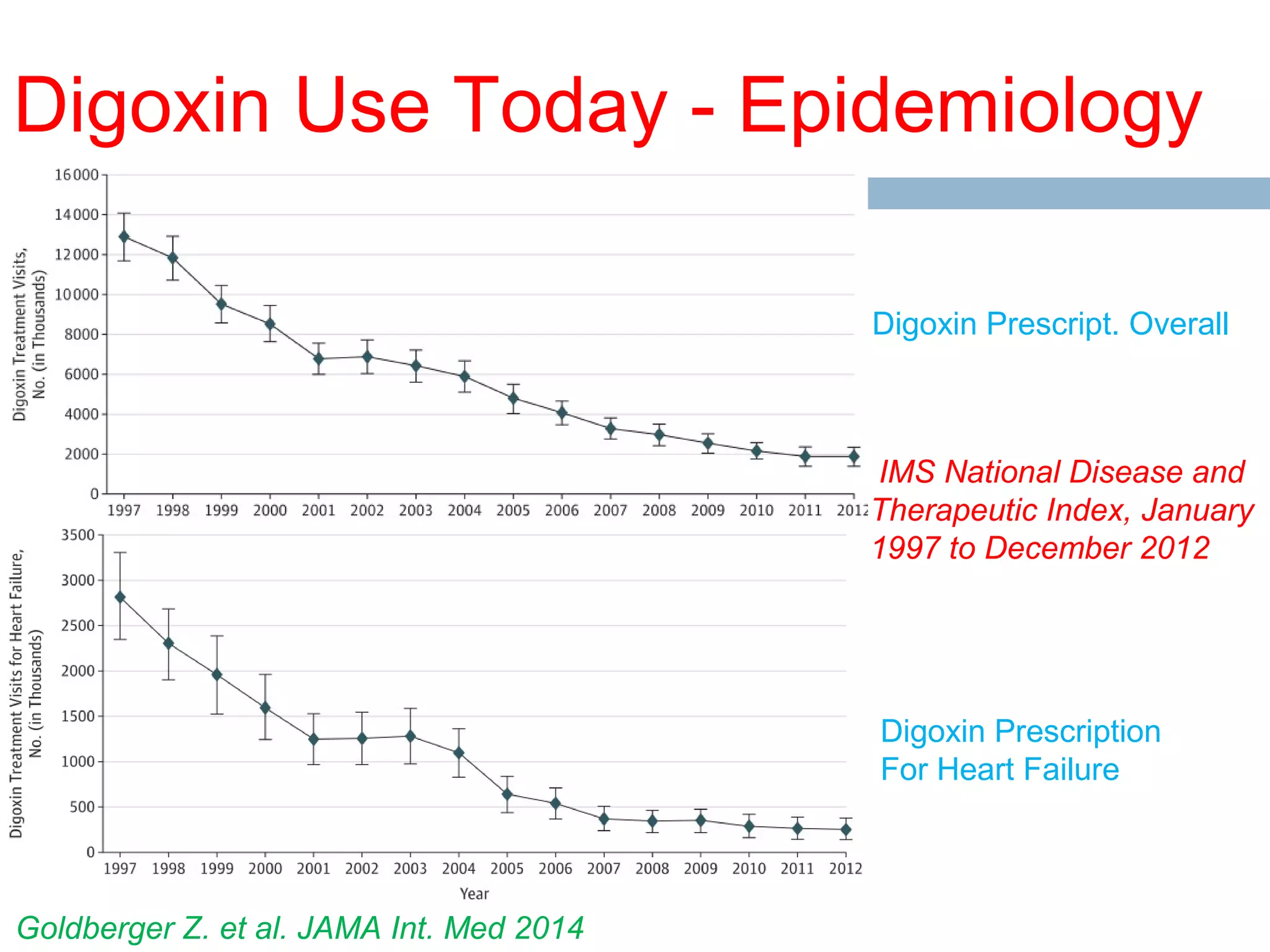
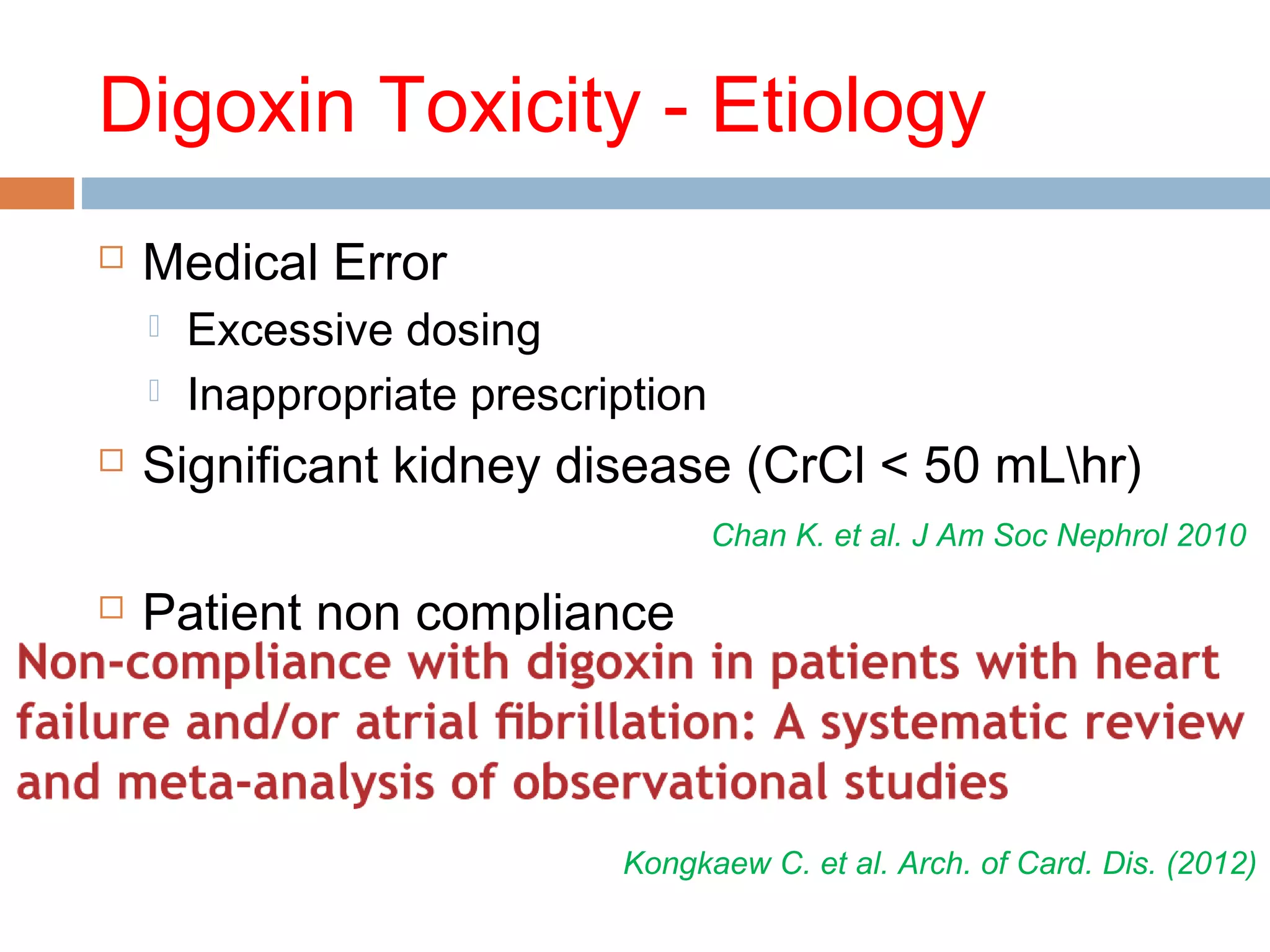
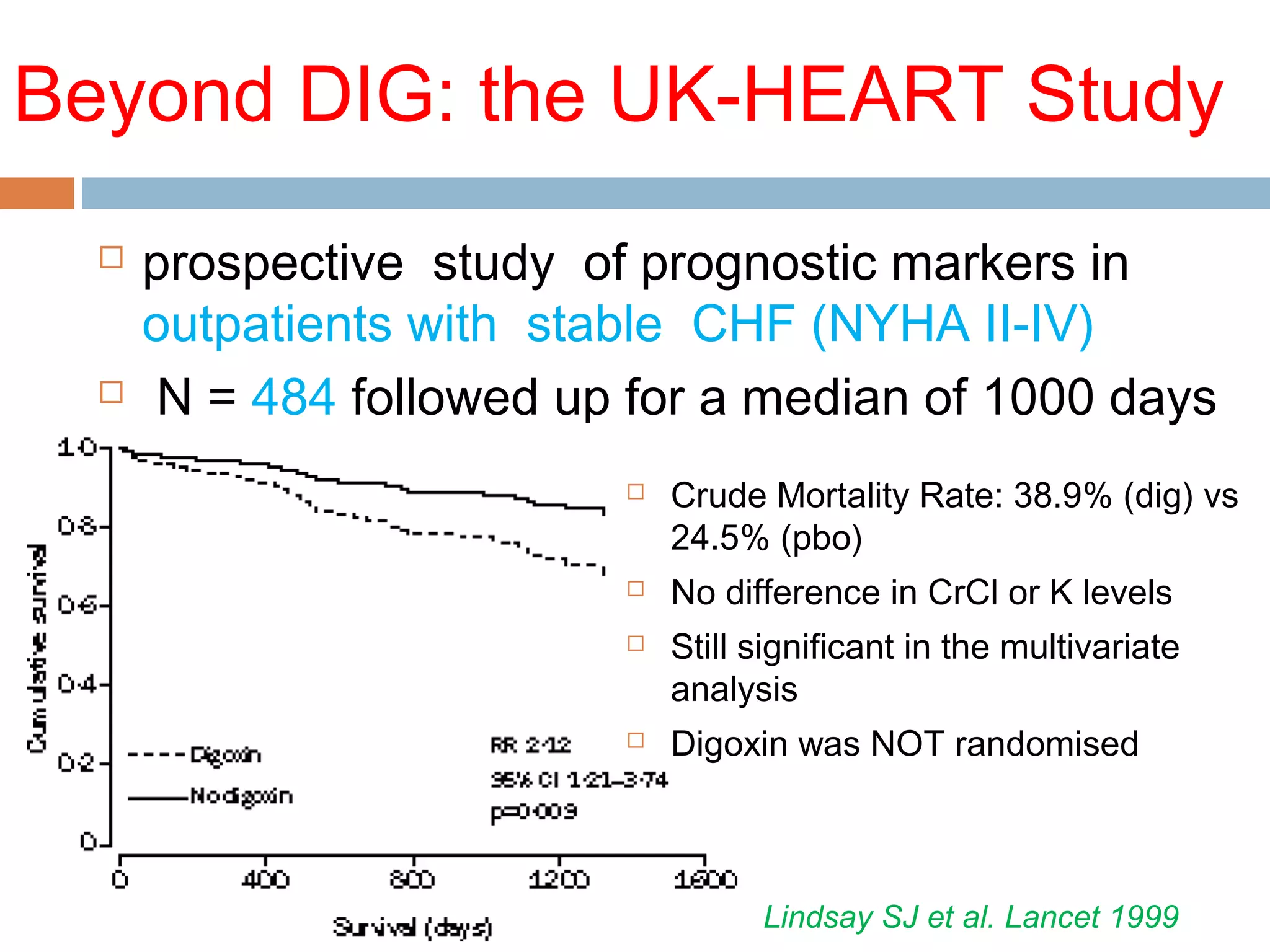
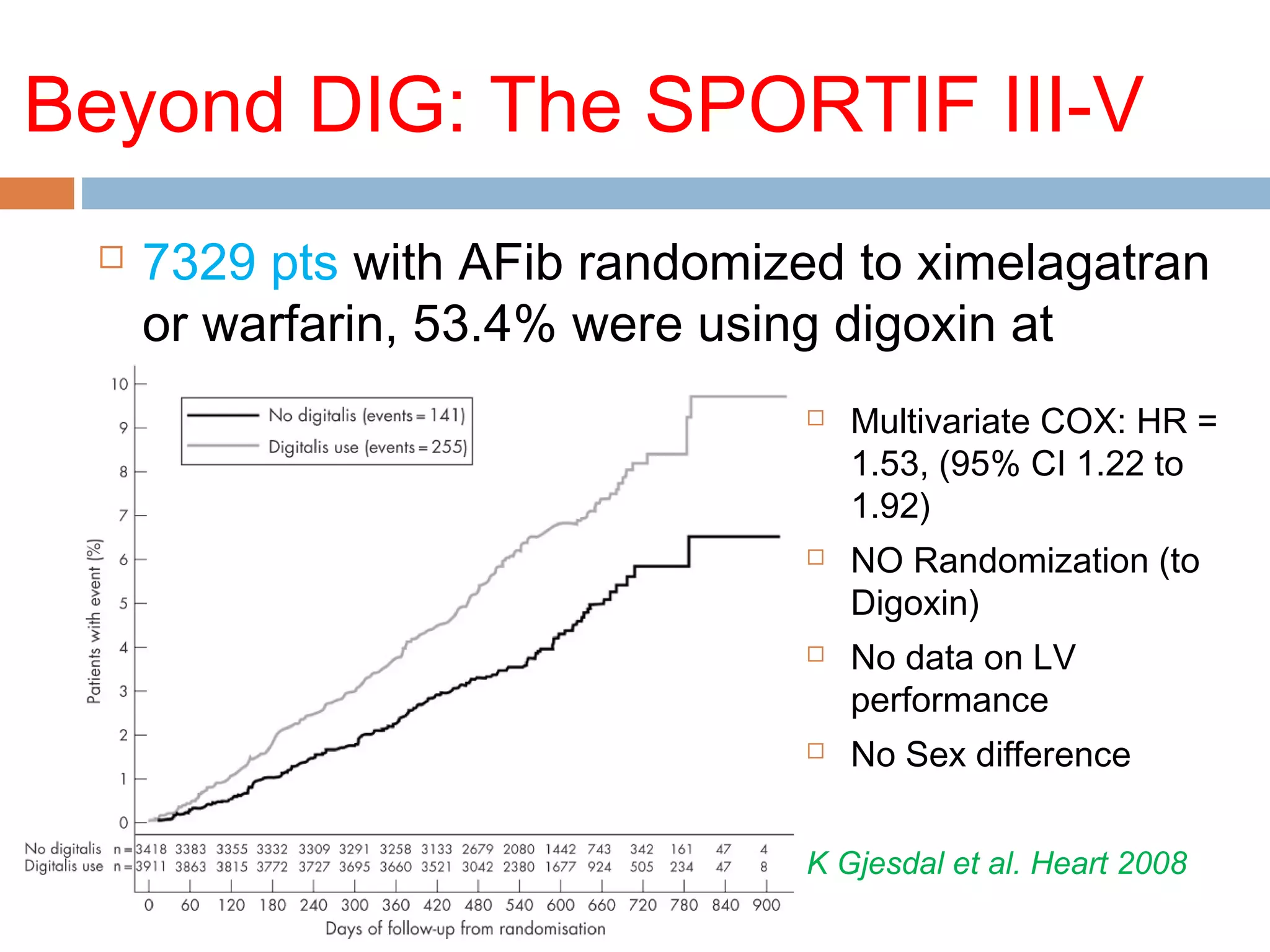
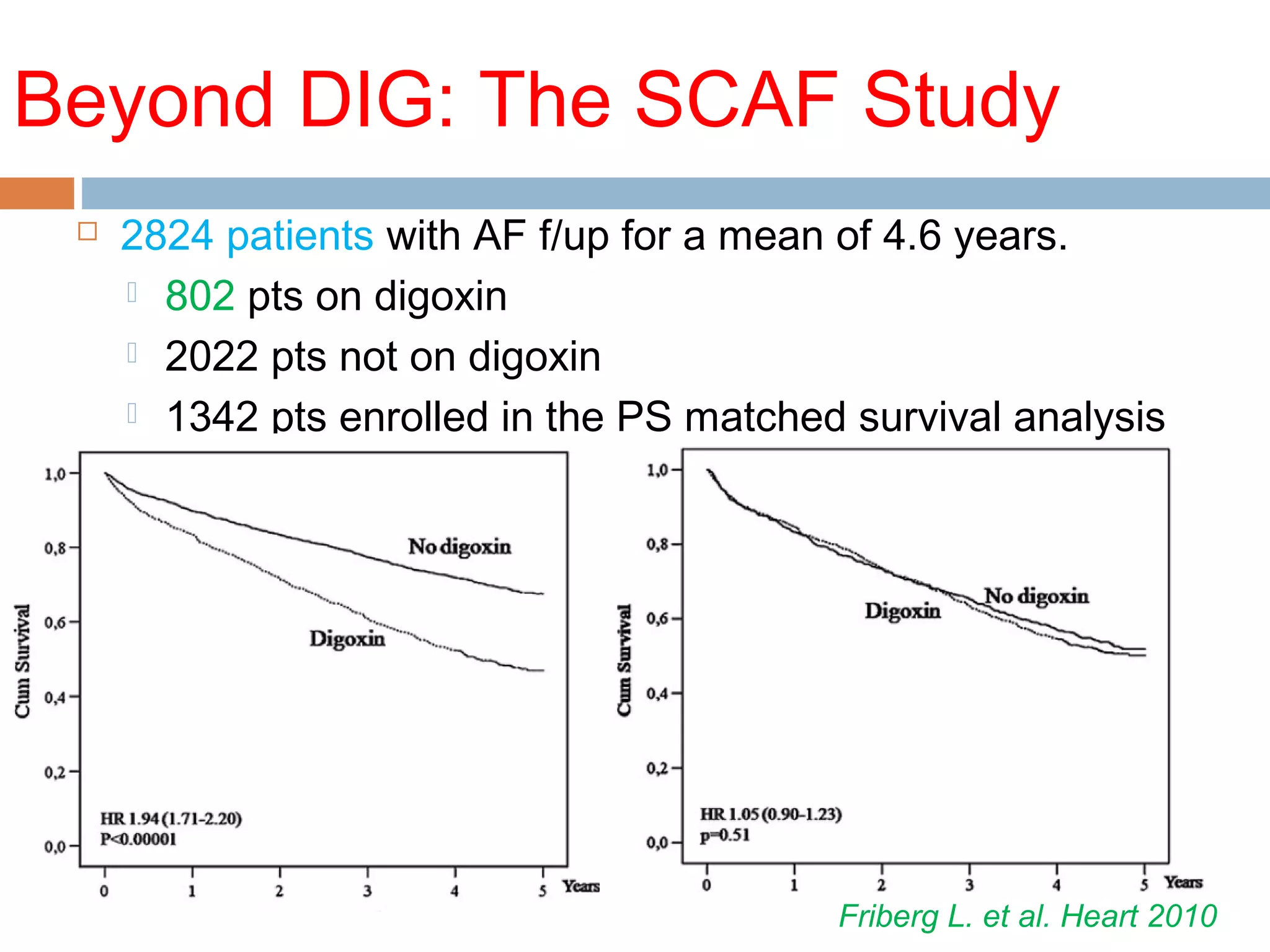
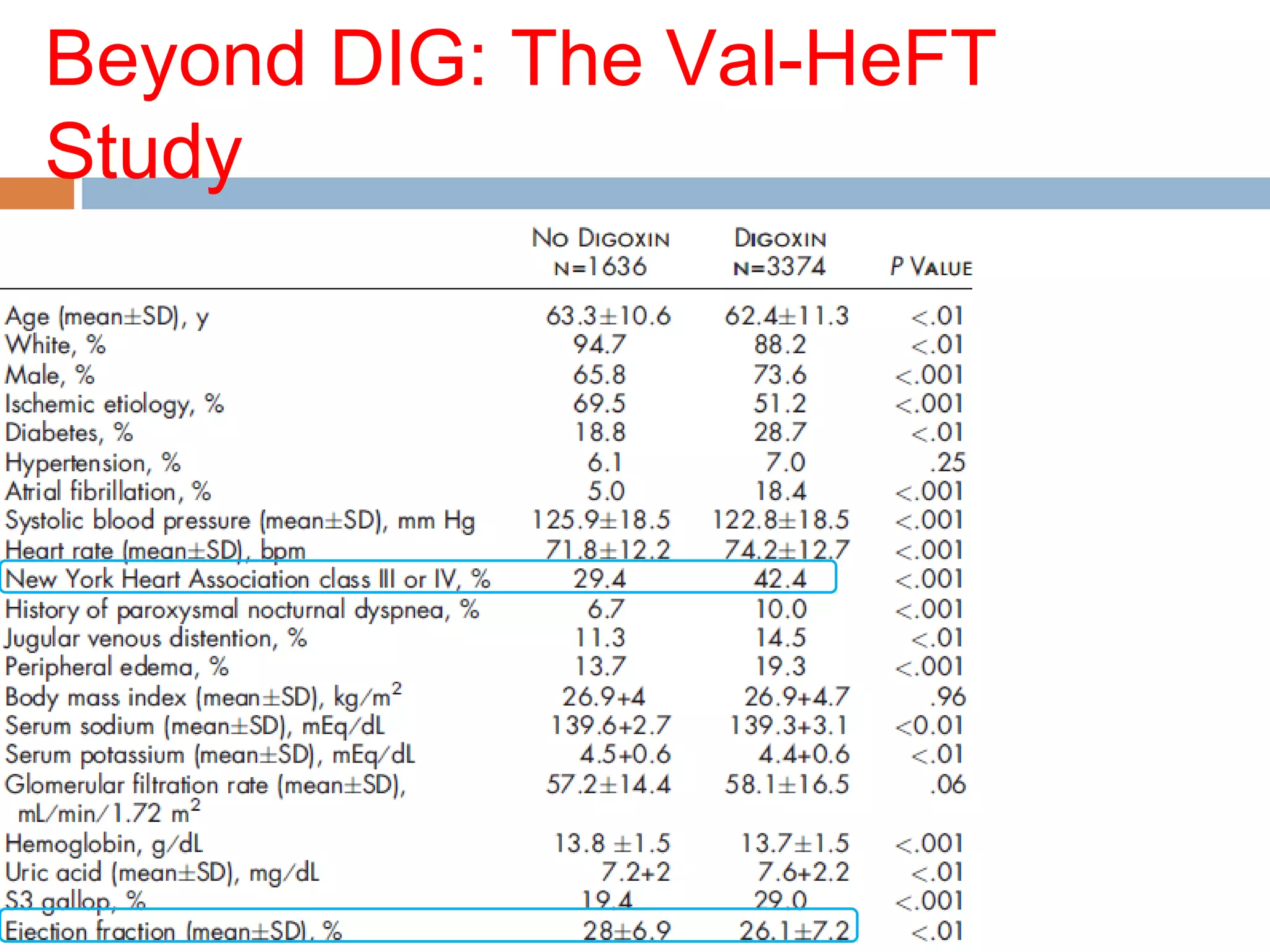
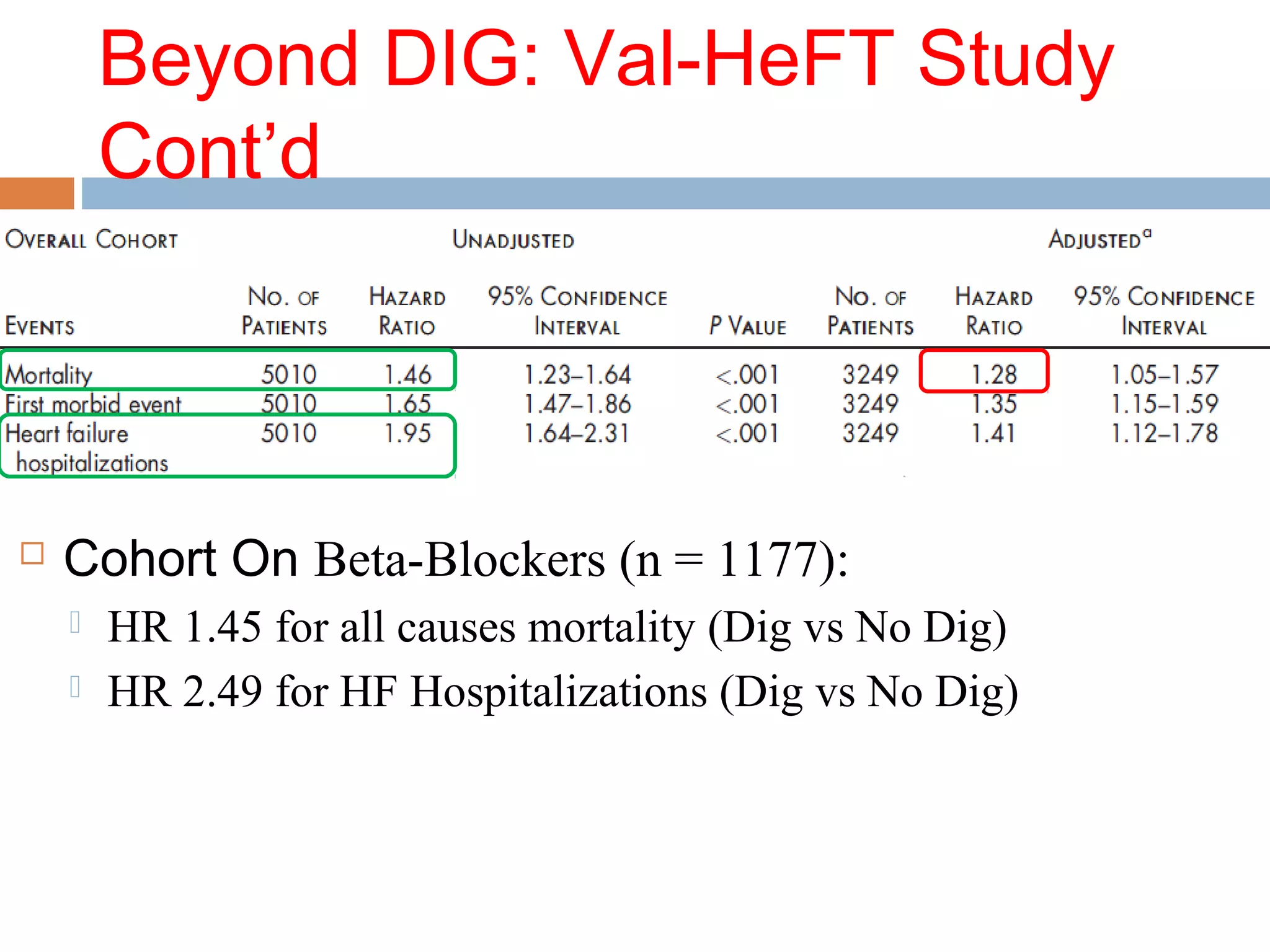
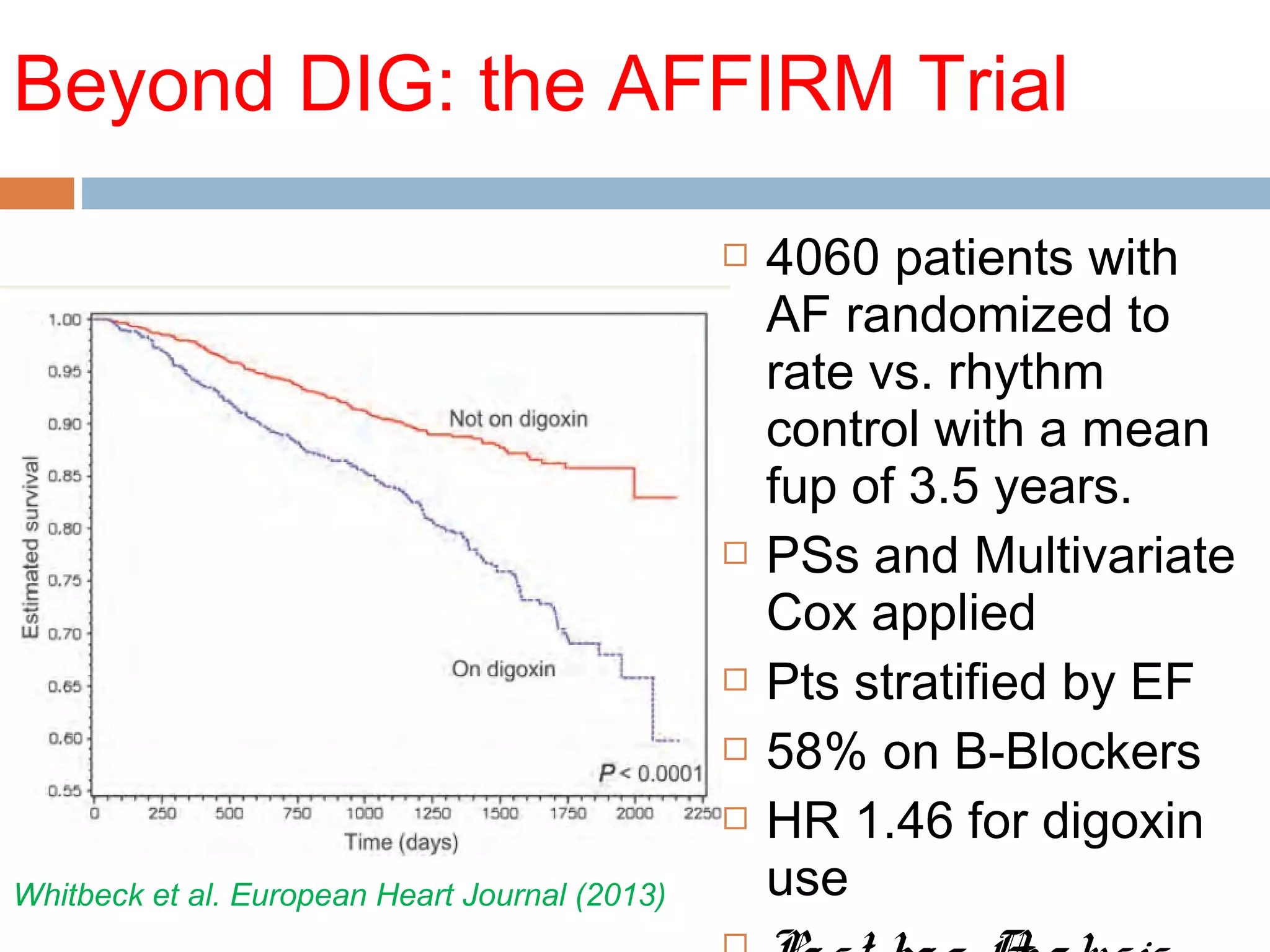
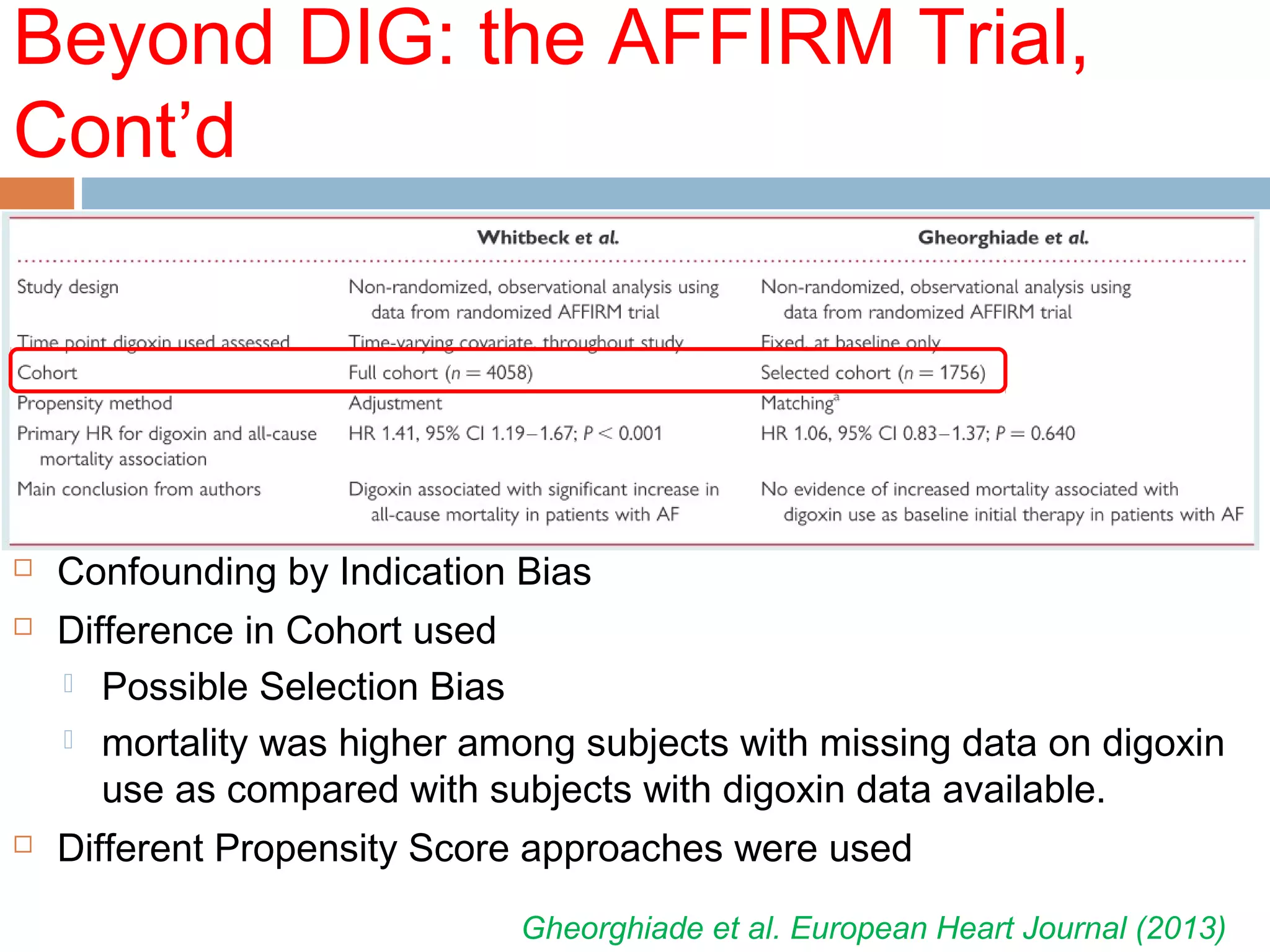
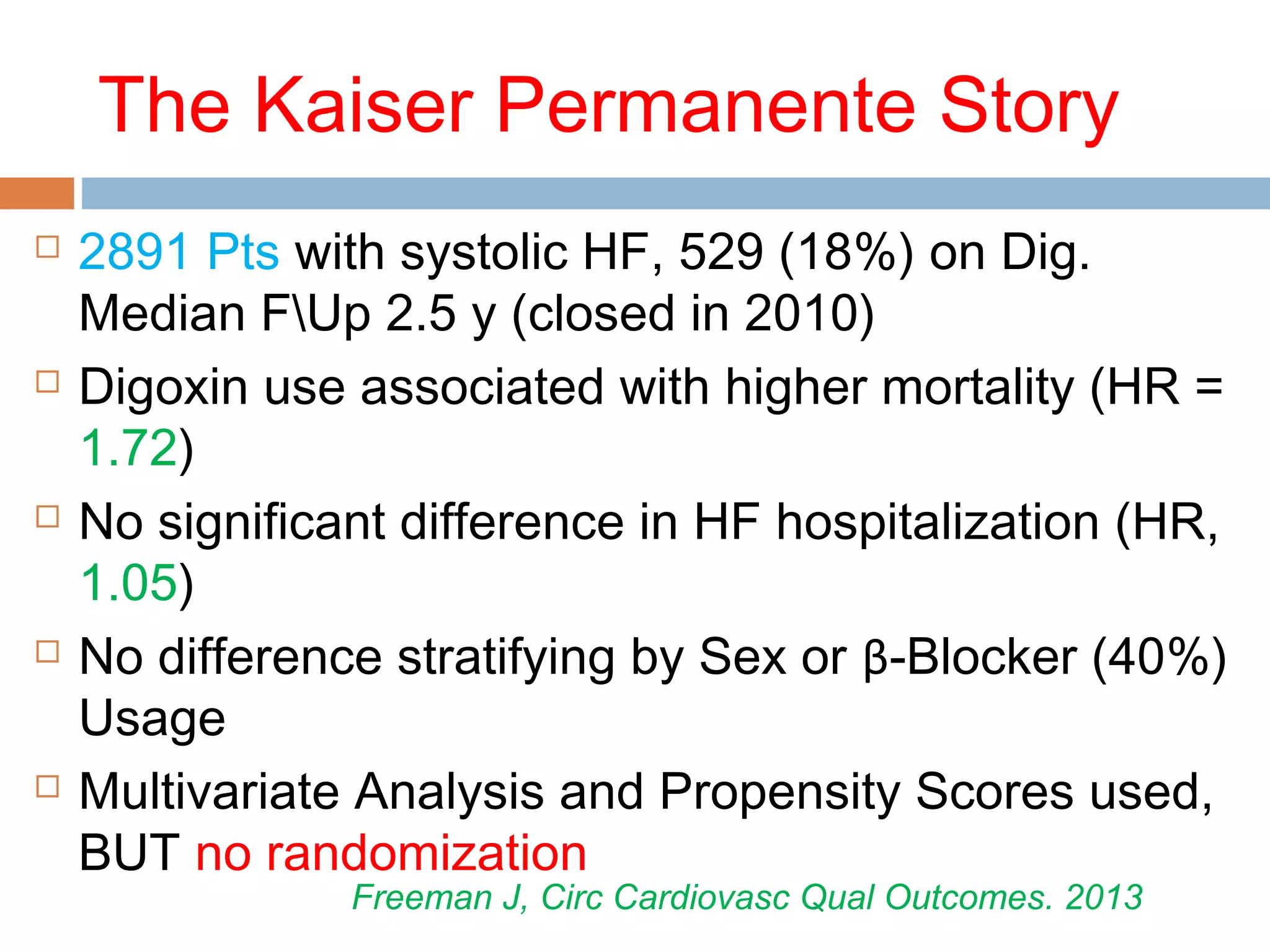
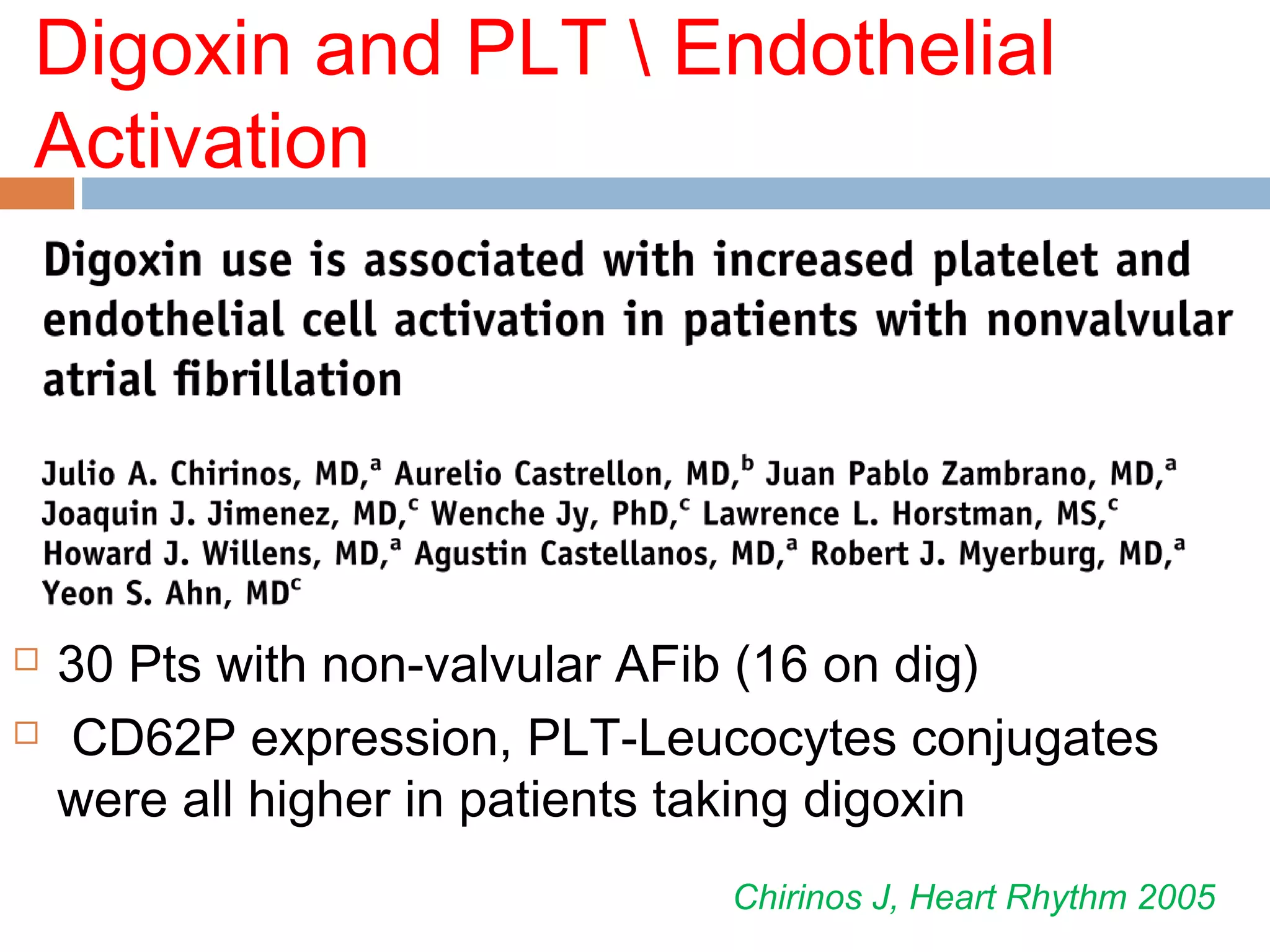
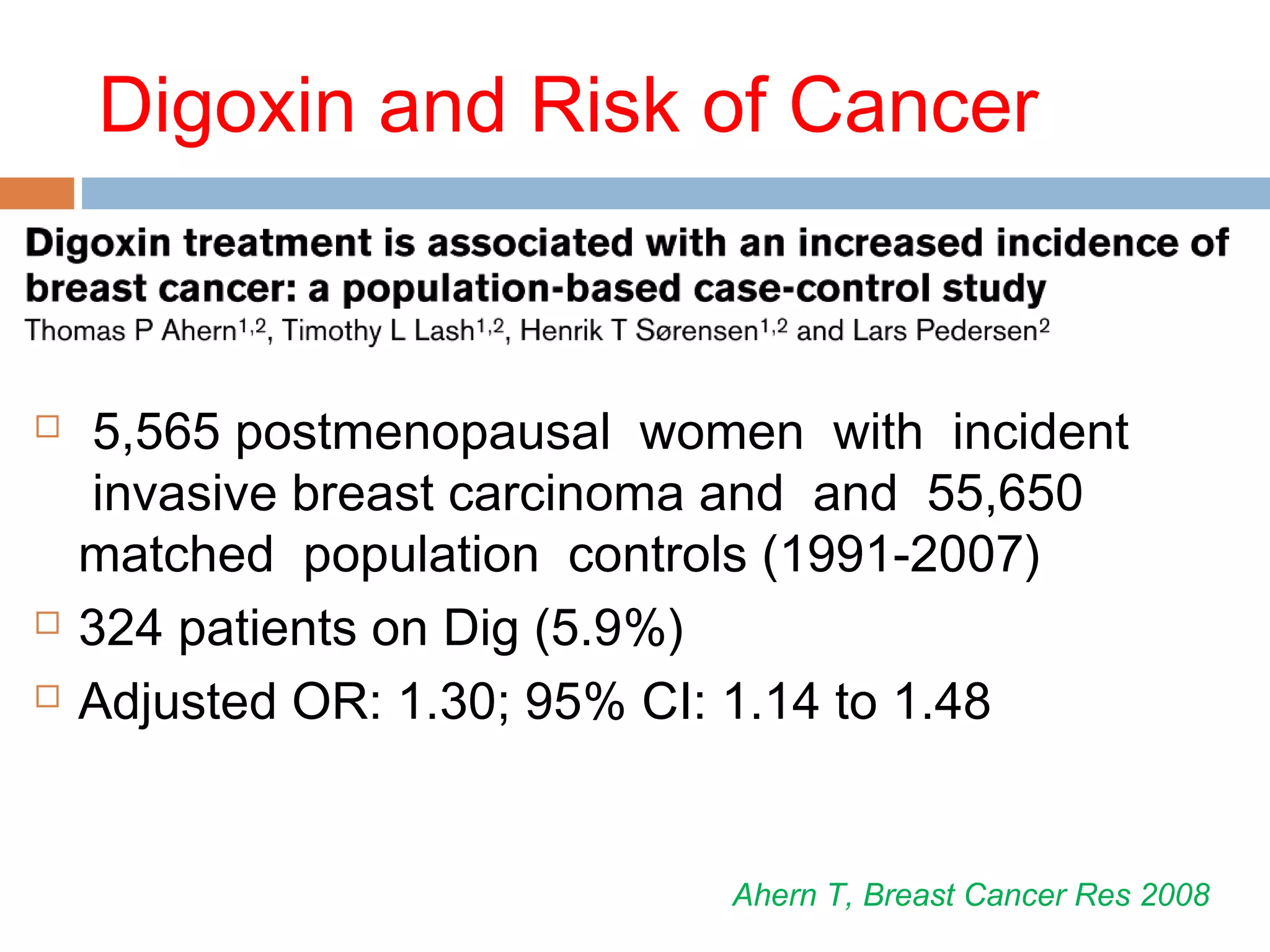
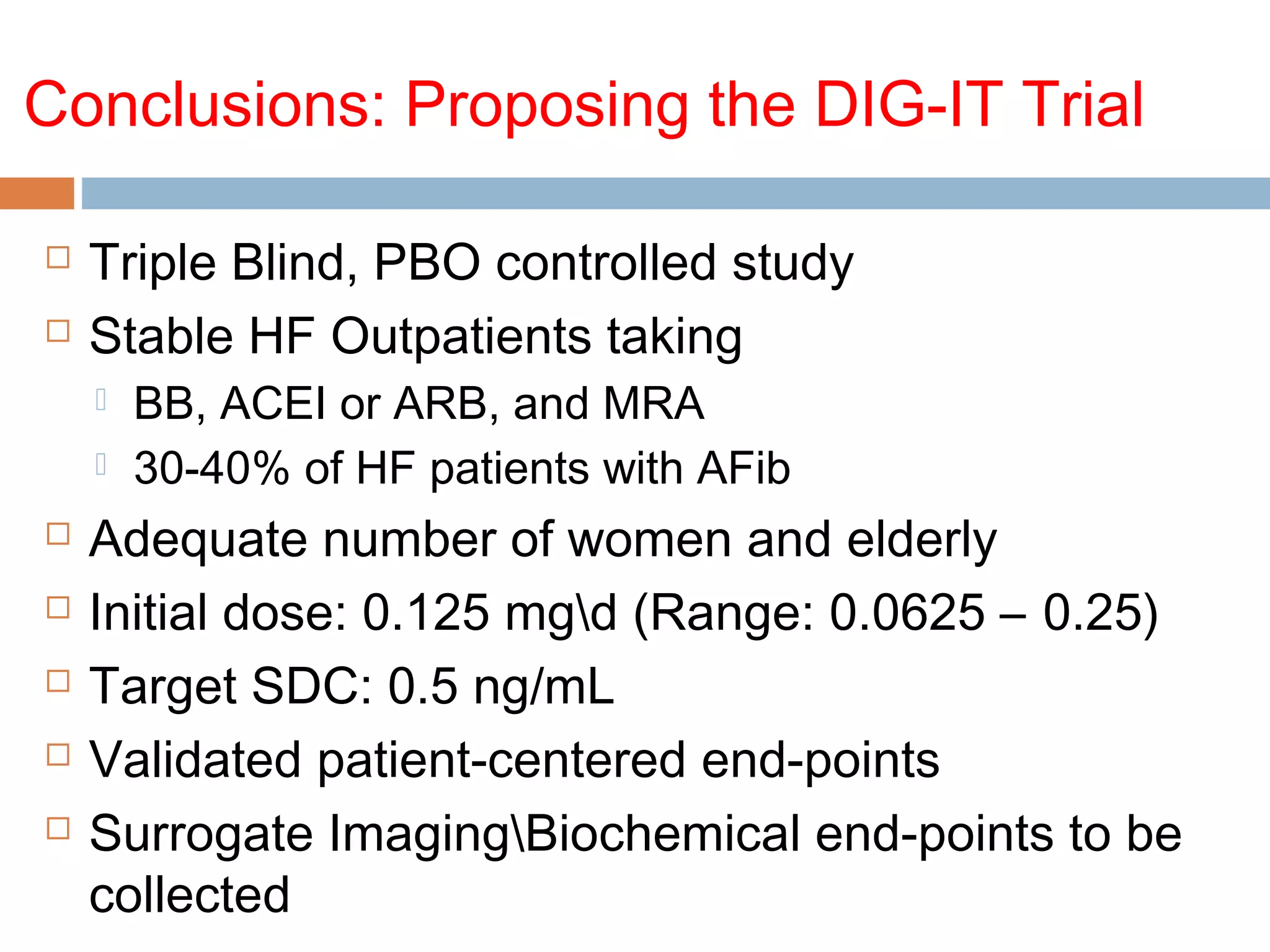
The document discusses the pharmacology, pharmacokinetics, and clinical usage of digoxin, focusing on its therapeutic effects, potential toxicities, and drug interactions. It highlights digoxin's role in managing atrial fibrillation and heart failure, emphasizing the importance of monitoring patient response and side effects due to its narrow therapeutic range. The text also addresses past studies regarding digoxin's efficacy and safety, along with recommendations for treatment protocols in cases of toxicity and heart failure management.


![DigiFAB Protocol
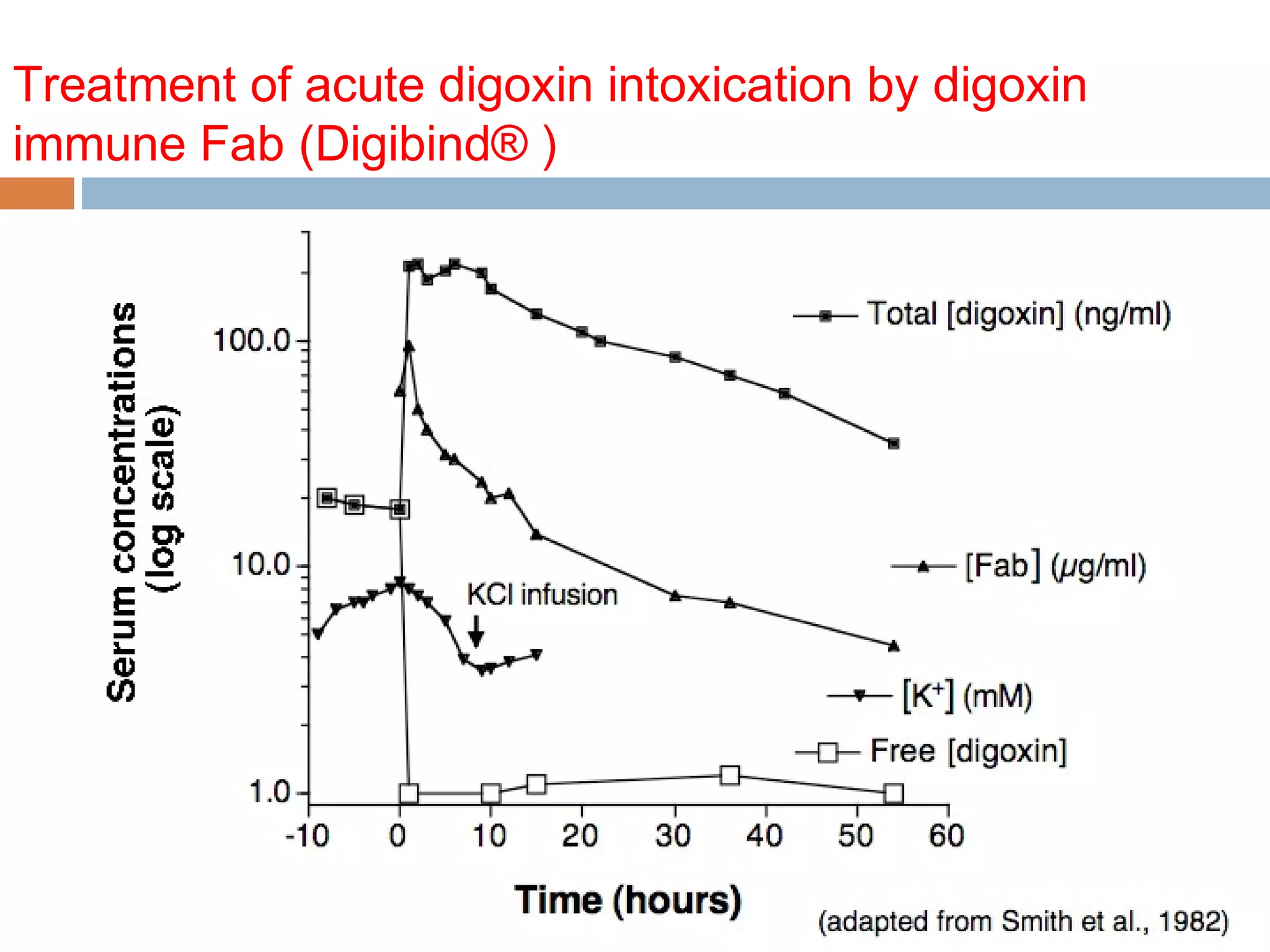
Fig. 1. Dosing recommendations for DSFab (Digibind or DigiFab). Infuse all doses over 30 minutes
through a 0.22-mm filter. If cardiac arrest is imminent, give via slow intravenous push. [dig]SS,
serum digoxin concentration (nanogram per milliliter) at steady state; F, esti- mated
bioavailability (if intravenous digoxin or digitoxin use 1, if digoxin tablets use 0.8); TBW,
total body weight. a Round number of vials upward. b If measurement in nanomole per liter,
multiply by 0.781. c If measurement in nanomole per liter, multiply by 0.765. d Inges- tions of
cardiac glycosides other than digoxin or digitoxin should be treated with empiric dosing
recommendations.](https://image.slidesharecdn.com/digbergamo2014-ii-140419143620-phpapp01/75/Digoxin-Toxicity-and-Trials-46-2048.jpg)