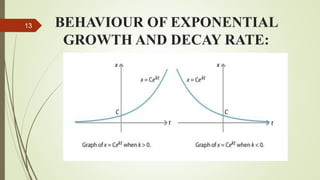
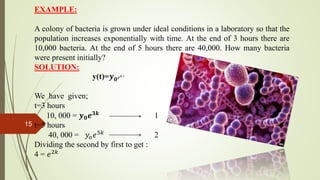
This document discusses differential equations and their applications. It defines ordinary and partial differential equations, and describes various types of ordinary differential equations including separable, homogeneous, non-homogeneous, exact, and linear equations. It also discusses exponential growth and decay models using differential equations. Examples are provided to illustrate radioactive decay modeling using half-life equations and carbon dating to determine the age of fossils.