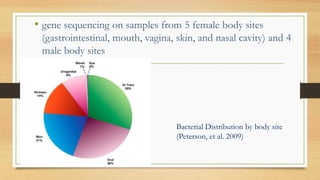
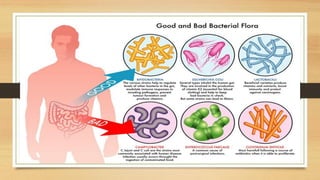
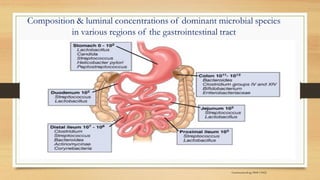
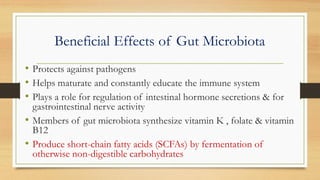
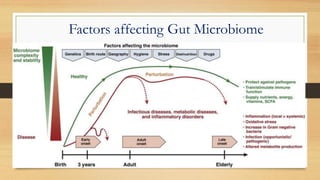
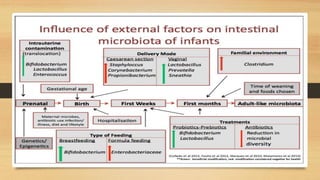
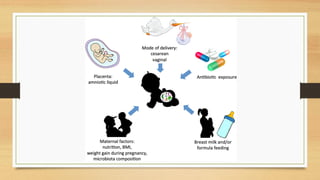
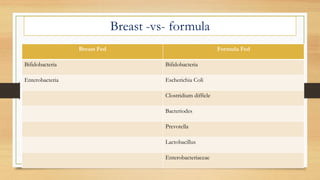
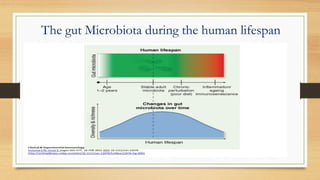
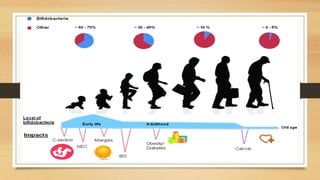
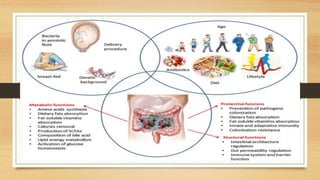
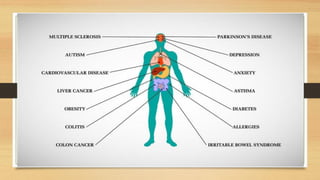
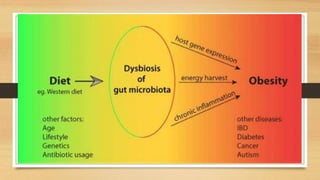
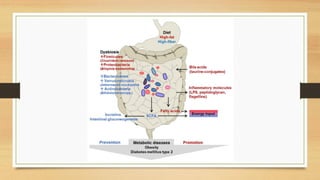
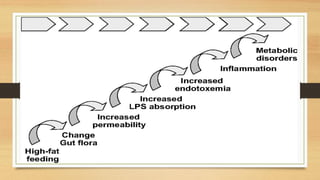
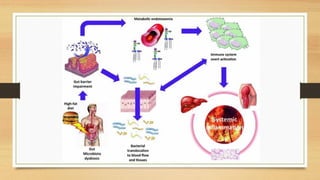
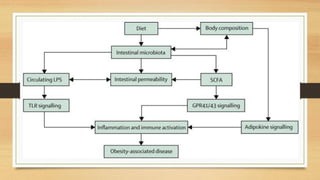
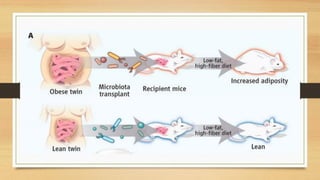
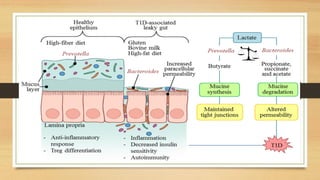
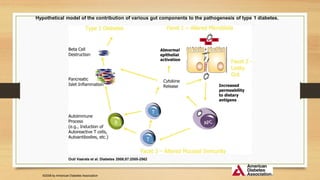
This document discusses the relationship between gut microbiota and diabetes. It begins by defining microbiota and describing the bacterial composition in different body sites. Environmental factors like breastfeeding, antibiotics, and diet impact the development of gut microbiota. Dysbiosis, or an imbalance in the normal microbiota, has been linked to various diseases like obesity, metabolic syndrome, and both type 1 and type 2 diabetes. A high-fat diet can cause dysbiosis and metabolic endotoxemia, increasing inflammation and insulin resistance. Probiotics, prebiotics, and dietary fiber may help maintain a healthy microbiota and prevent diabetes. Both genetic and environmental factors contribute to the complex relationship between the gut, immune system, and development of