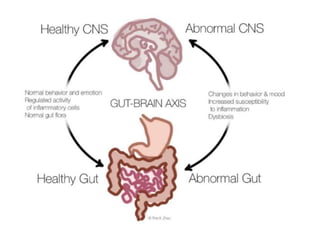
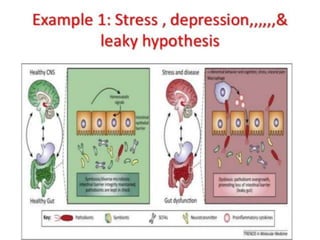
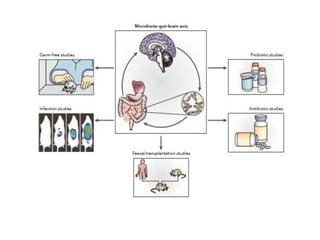
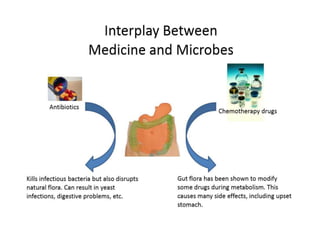
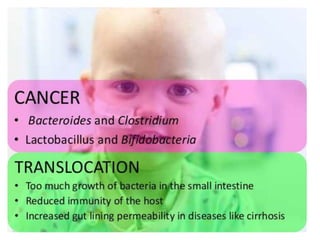
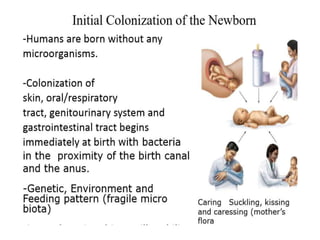
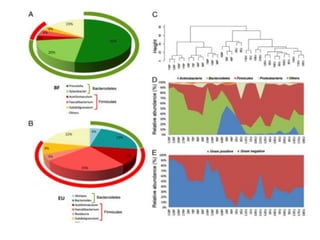
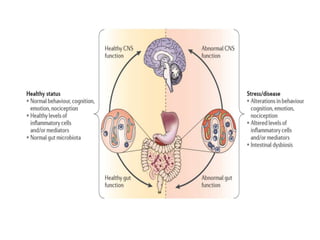
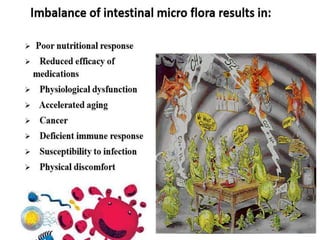
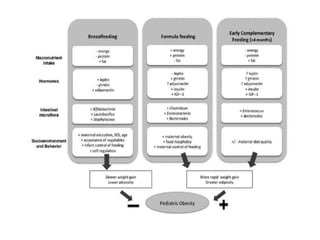
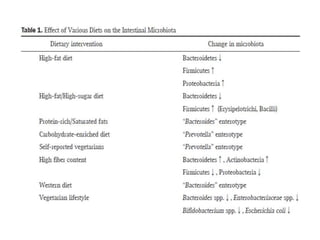
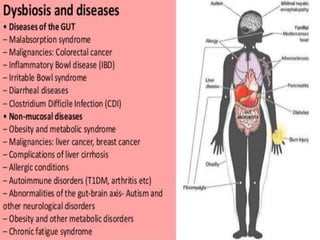
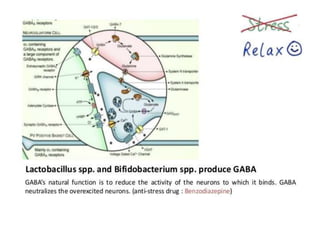
The document discusses the significant role of the gut microbiome in the immune system, highlighting factors such as diet, stress, and antibiotics that can alter its composition. It emphasizes the gut-brain axis and its influence on neurological functions, including mood and behavior, and examines the impact of gut microbiota on various health conditions like obesity and autism. Additionally, it addresses the therapeutic potential of probiotics and the effects of antibiotics on gut health.