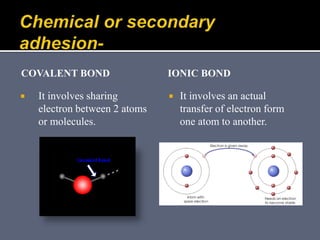
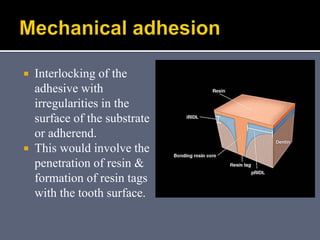
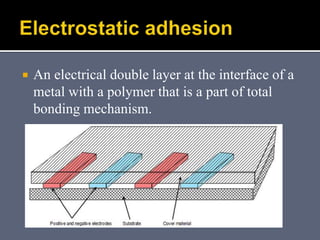
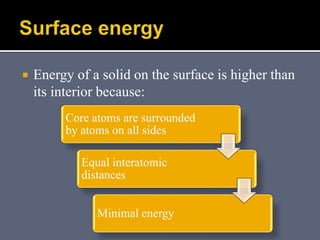
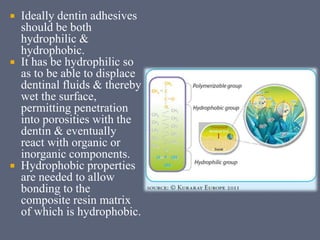
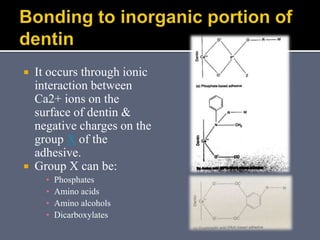
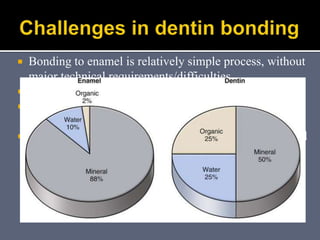
The document discusses the history and development of dental bonding systems. It describes the key differences between first, second, and third generation bonding agents. First generation agents from the 1960s produced weak bonds of 2-3 MPa and had high failure rates. Second generation agents from the 1970s-1980s left the smear layer intact and achieved bonds of 4.5-6 MPa. Third generation "total-etch" systems from the 1990s removed the smear layer prior to bonding and produced stronger bonds of 16-26 MPa approaching that of enamel. The three-step approach of conditioning, priming, and applying adhesive resin was developed to strongly bond to both enamel and dentin.