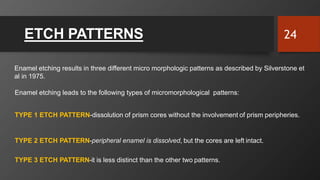
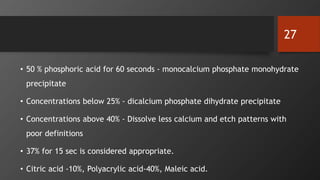
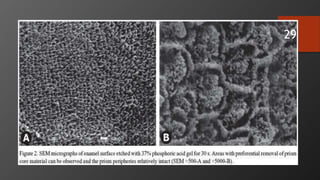
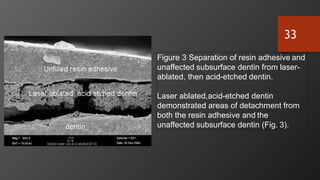
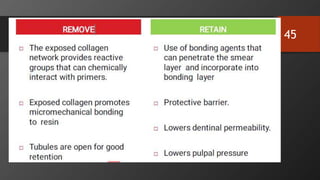
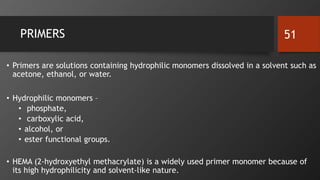
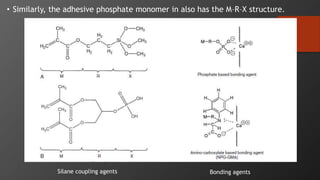
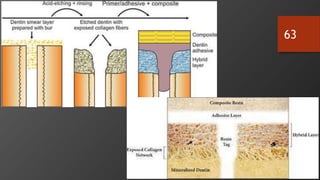
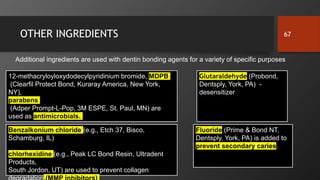
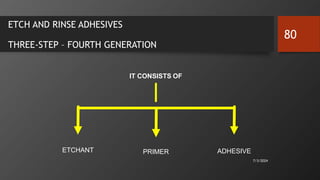
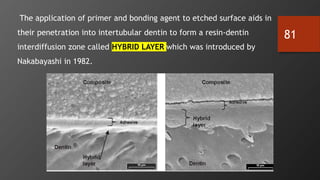
The document discusses dentin bonding agents in restorative dentistry, focusing on their history, mechanisms, and factors influencing adhesion. It outlines the evolution of bonding techniques from the 1950s to contemporary methods and highlights the challenges of achieving effective bonds to dentin due to its unique structure and the presence of the smear layer. Key topics include the importance of surface energy, wetting, and the role of various bonding systems in enhancing adhesion to both enamel and dentin.




![HISTORY
• Research into bonding agents for attachment of resins to tooth structure was
started in the early 1950s.
• In 1949, Hagger, a Swiss chemist attempted first to develop an adhesive
system for bonding acrylic resin to the tooth structure, [acidic glycerophosphoric
acid dimethacrylate].
• In 1949, a commercial product Sevriton cavity seal was then marketed.
6](https://image.slidesharecdn.com/7-240703091559-b5f0e26a/85/7-DENTIN-BONDING-AGENTS-FOR-COMPOSITES-pptx-6-320.jpg)













![ACID ETCH TECHNIQUE-
ENAMEL ETCHING
• Michael Buonocore (1955).
• He found that an acrylic restorative material placed on the micromechanically roughened
surfaces greatly increased in the resin–enamel bond strength (~20 megapascals [MPa])
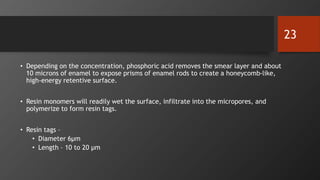
• Phosphoric acid, is still the most widely used etchant today for bonding to both enamel
and dentin.
22](https://image.slidesharecdn.com/7-240703091559-b5f0e26a/85/7-DENTIN-BONDING-AGENTS-FOR-COMPOSITES-pptx-22-320.jpg)




























![INITIATORS
• Polymerization can be initiated either
• through a photoinitiator system consisting of a photosensitizer (e.g.,
camphorquinone) and an initiator (e.g., tertiary amine),
• through a self-cure system that includes a chemical initiator (e.g., benzoyl
peroxide [BPO]),
• or through a dual-cure initiator system.
65](https://image.slidesharecdn.com/7-240703091559-b5f0e26a/85/7-DENTIN-BONDING-AGENTS-FOR-COMPOSITES-pptx-65-320.jpg)


































![106
Comparative analysis of bond strength and microleakage of newer generation bonding agents to enamel and
dentin: Anin vitro study
• Nishmitha Hegde, Shruthi Attavar, Mithra N Hegde, Nidarsh D Hegde Department of Conservative Dentistry
and Endodontics, A.B. Shetty Memorial Institute of Dental Sciences,Mangalore, Karnataka, India
• Journal of conservative dentistry 2020
Resin bonded with self-etch G-Premio Bond used in selective etch technique showed the highest
BS and resistance to ML.
• G-Premio Bond (GC Asia Dental Pte. Ltd.,), a universal bonding agent, 8th generation provides
outstanding durability, compatible with total-etch self-etch, and selective etch techniques.
• A unusual combination of three functional monomers (4methacryloyloxyethyltrimellitic acids
[4-MET], MDP and MDTP), excluding HEMA,
• It ensures stability and exceptional BS not only for dental tissue but also for all indirect
substrates, including precious and non-precious metals, composites, alumina, and zirconia for
repair cases.
• This adhesive system was bonded with G-ænial Sculpt, a nanohybrid composite.](https://image.slidesharecdn.com/7-240703091559-b5f0e26a/85/7-DENTIN-BONDING-AGENTS-FOR-COMPOSITES-pptx-106-320.jpg)







