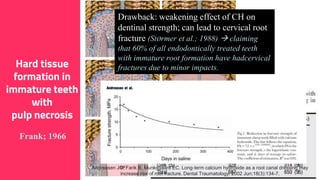
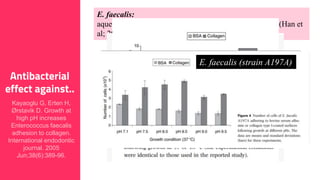
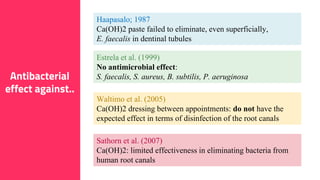
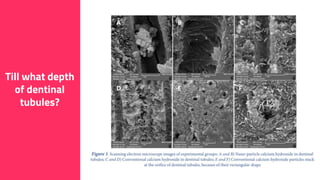
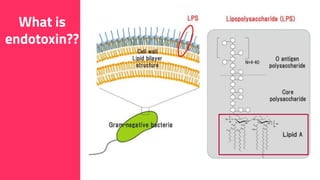
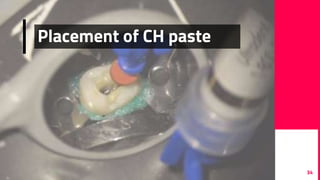
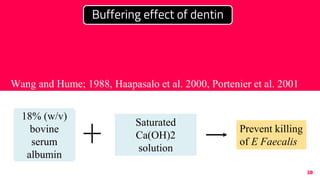
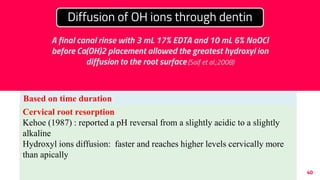
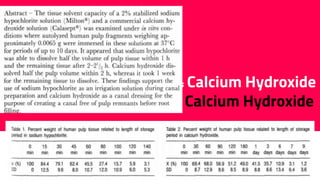
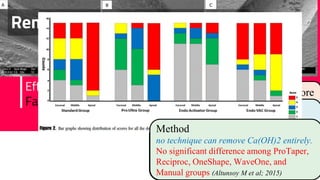
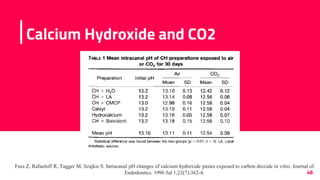
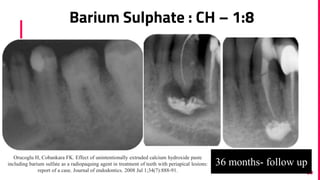
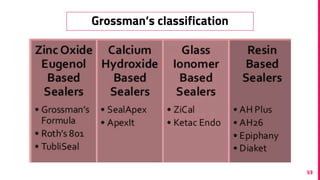
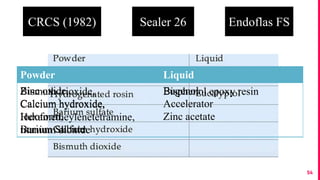
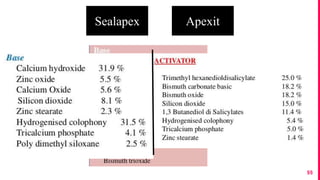
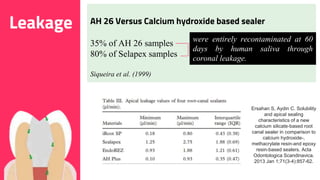
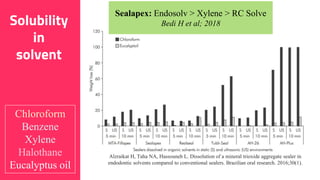
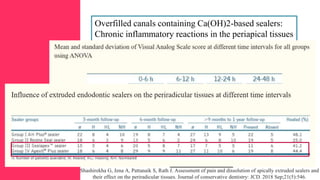
The document discusses the use of calcium hydroxide in endodontics, including its role as an intracanal medicament and root canal sealer. It covers various aspects such as its mechanism of action, clinical applications, effects on dentin, and its comparison with other materials like MTA. In addition, it addresses the antimicrobial efficacy, placement techniques, and considerations for its removal from the canal.