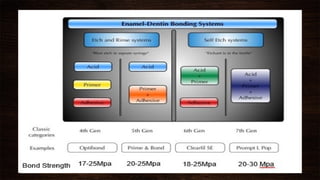
The document presents a comprehensive overview of dental bonding, including its historical development, mechanisms, and various types of adhesive systems. It details the evolution from early bonding agents to contemporary universal adhesives, as well as the scientific principles behind adhesion and bonding to dental tissues like enamel and dentin. Key factors influencing bonding stability and techniques for optimizing adhesion in dental procedures are also explored.