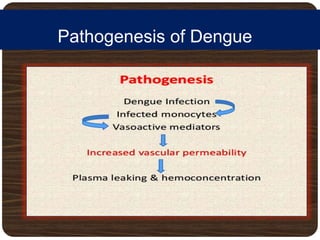
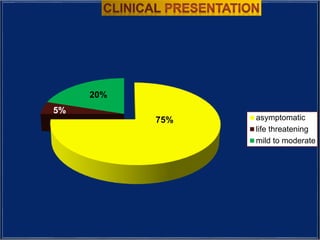
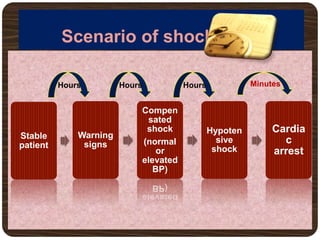
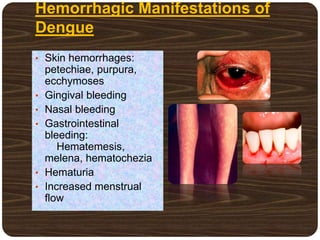
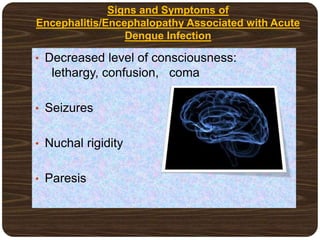
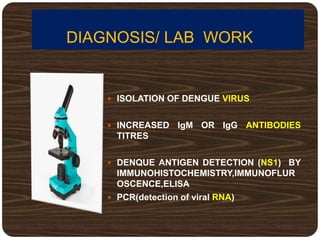
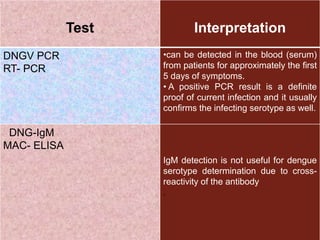
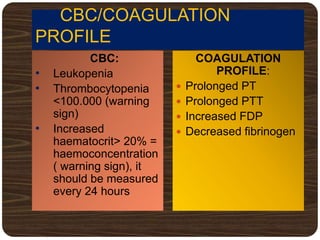
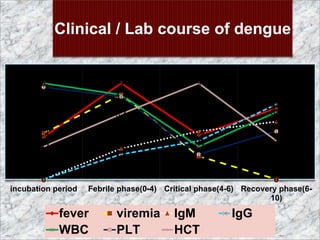
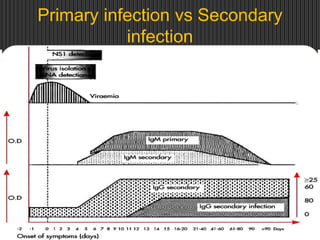
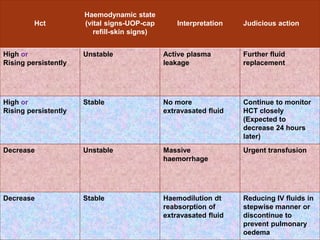
The document provides an overview of dengue fever, including its history, global burden, virus, vector, transmission, pathogenesis, clinical manifestations, diagnosis, and management. Some key points:
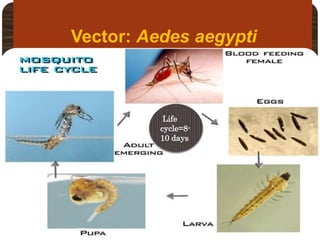
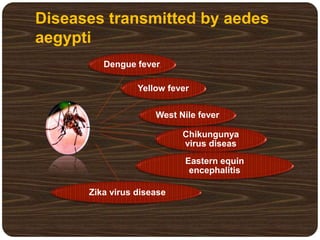
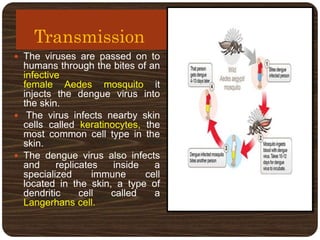
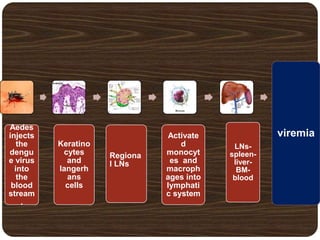
- Dengue is caused by the dengue virus and transmitted by Aedes aegypti mosquitoes. It ranges from a self-limiting fever to life-threatening dengue hemorrhagic fever/shock syndrome.
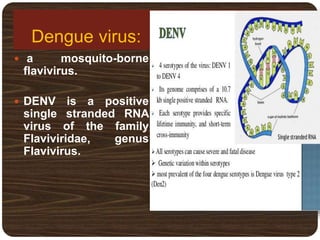
- There are 4 serotypes of the virus. Secondary infection with a new serotype increases the risk of severe disease.
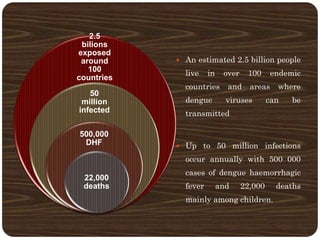
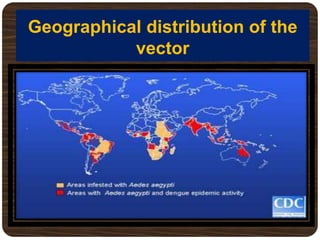
- Over 2.5 billion people in over 100 countries are at risk annually, with up to 50 million infections and 22,000 deaths mainly in children.
-