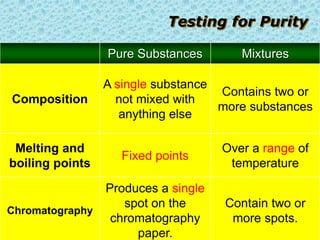
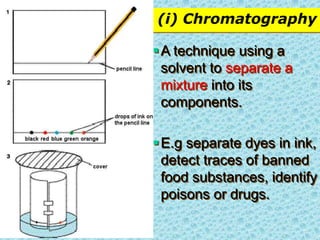
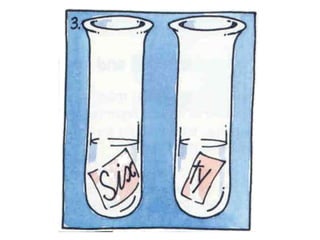
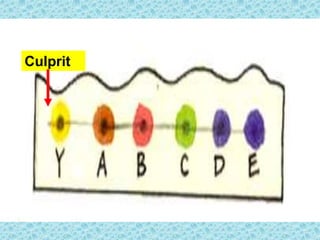
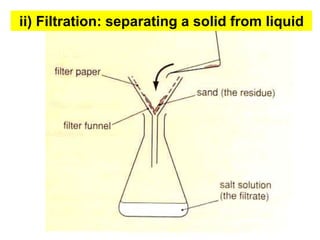
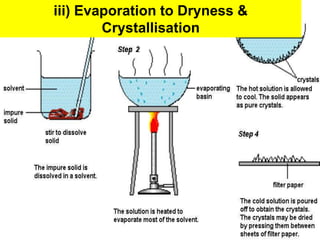
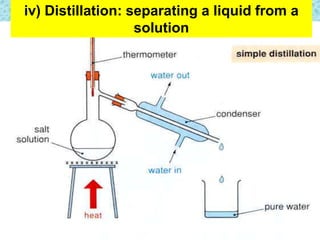
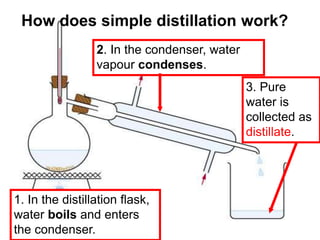
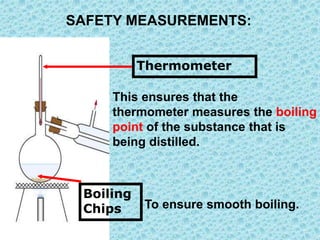
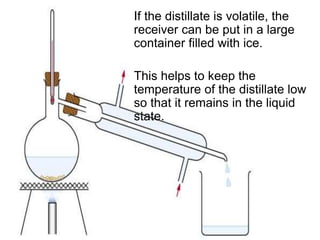
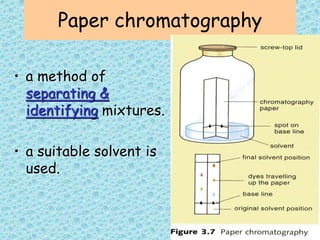
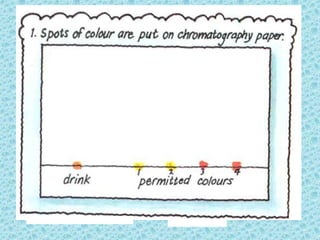
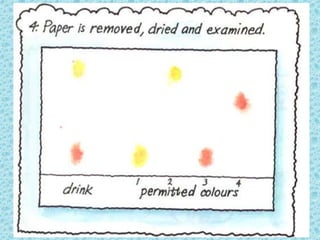
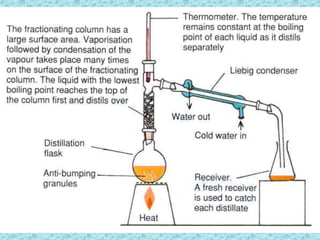
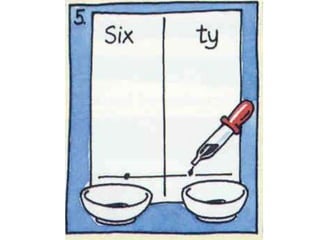
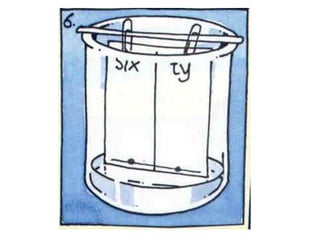
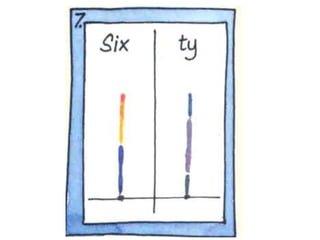
This chapter discusses separation techniques including chromatography, filtration, crystallization, and distillation. Chromatography involves using a solvent to separate mixtures into individual components by exploiting differences in how substances migrate across a medium. Filtration separates solids from liquids by passing a mixture through a filter. Crystallization and distillation are other common separation methods. The document provides detailed procedures and diagrams for each technique.