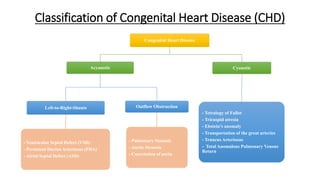
1. Ebstein's anomaly is a rare congenital heart defect where the tricuspid valve is displaced downward into the right ventricle, causing obstruction of blood flow.
2. This leads to a shunt of deoxygenated blood from the right to left atrium, causing cyanosis.
3. Surgical options include tricuspid valve repair or replacement, closure of intra-atrial communications, and reduction of the enlarged right atrium and right ventricle. The goal is to improve valve function and cardiac output while eliminating arrhythmias.