The document discusses cryogenics, detailing temperature ranges, material characteristics, and various applications, including superconductivity, LNG transport, and food preservation. It highlights how materials behave at cryogenic temperatures, mentioning increases in tensile strength but decreases in ductility, which can lead to brittle fractures. The text also outlines historical advancements in cryogenic technology and standards relevant to its engineering and application.
![CRYOGENICS - In Brief
(JGC Annamalai)
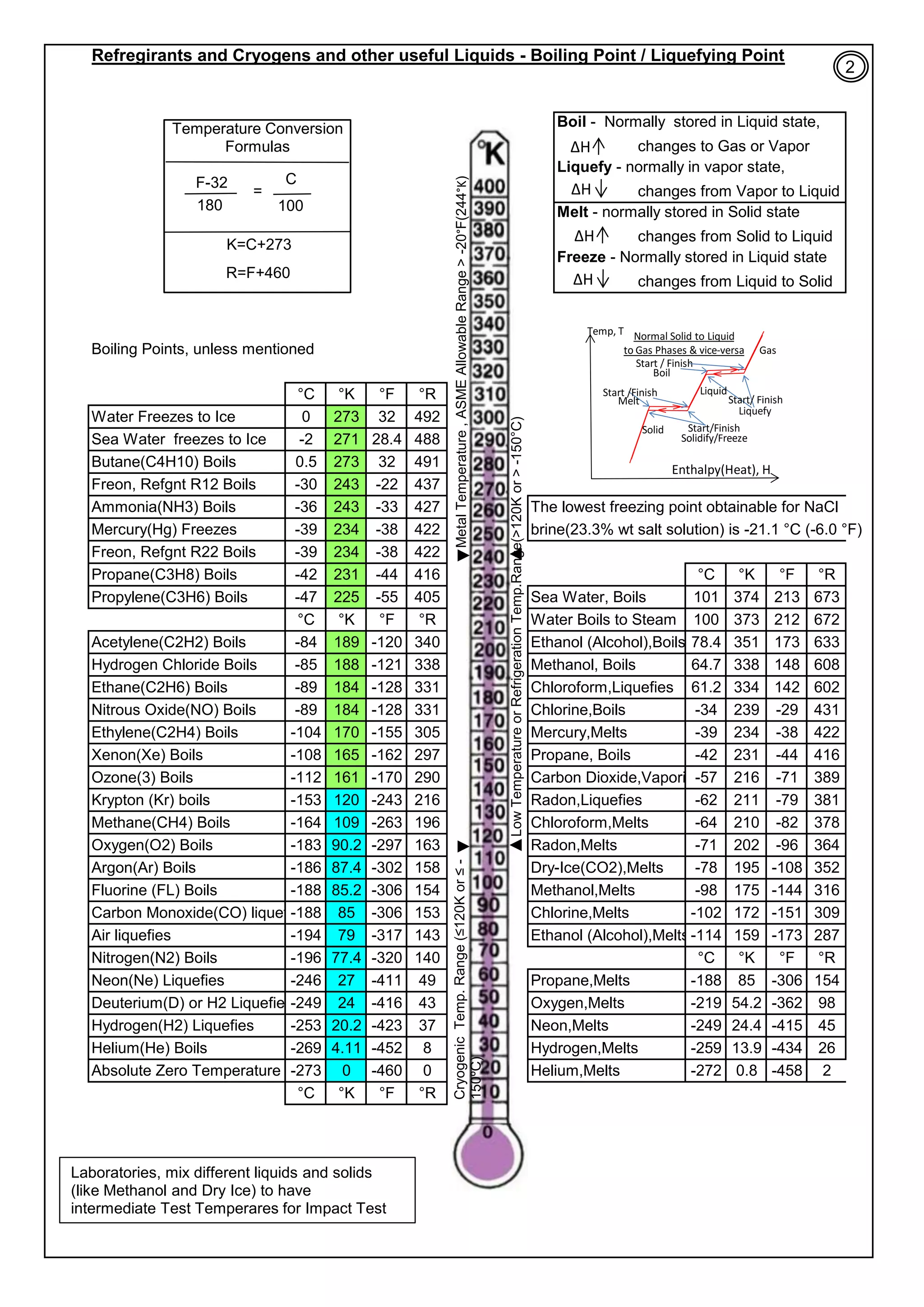
Normal or accepted range of cryogenic is from Absolute Zero to 120ºK (
‐
-273ºC to
‐
-150ºC).
The cycle is repeated till we achieve the cryogenic temperatures.
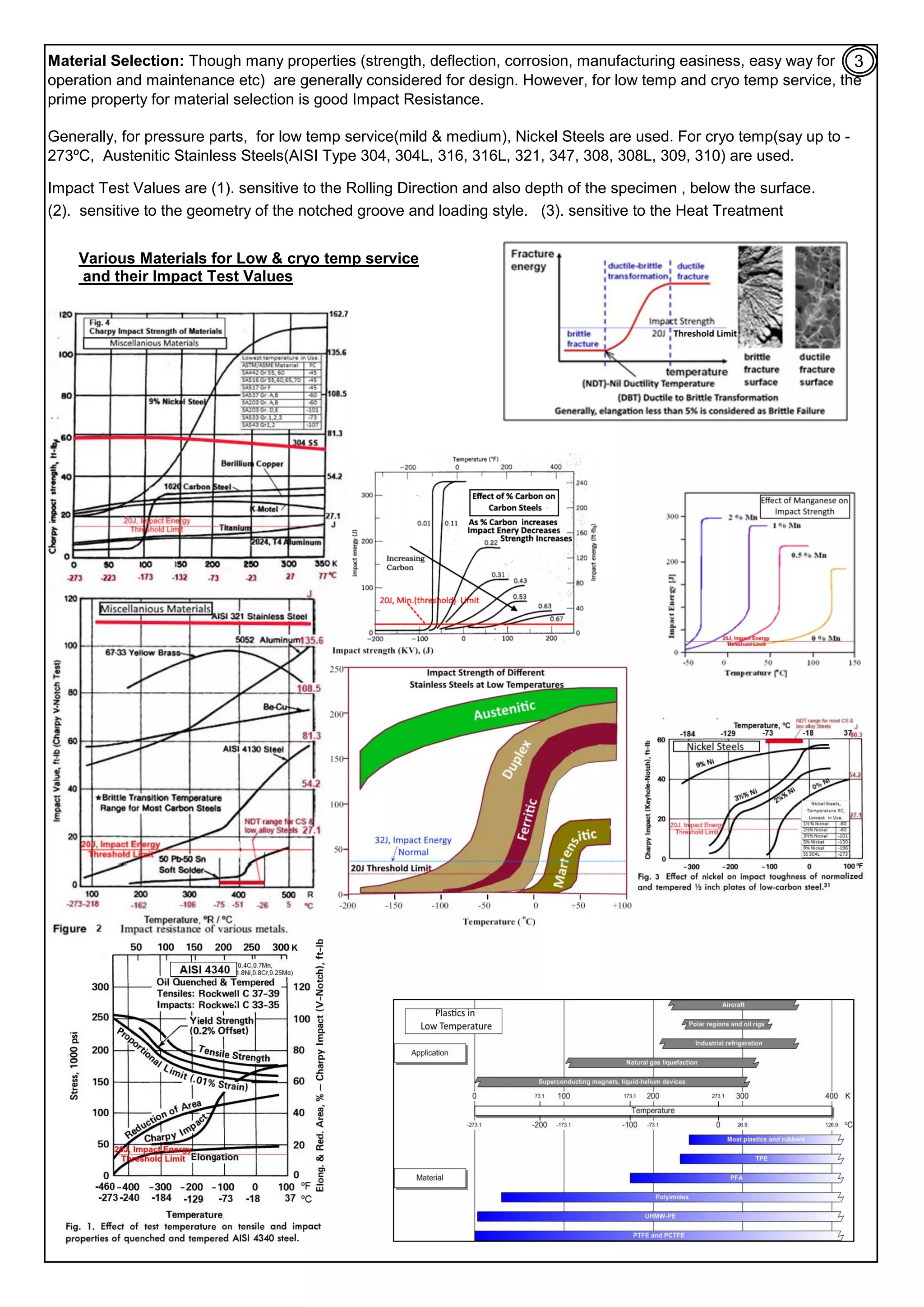
fracture study, better materials and improvements on Toughness / Impact Energy.
Application of Cryogenic Technology:
(C). ASME Sec VIII code permits material to use at temperature below -20ºF(
‐
- 29ºC)
only if the material is tested at the operating temperature and passes the minimum
requirements for impact resistance.
(D).Titanic Ship , Liberty Ships, Pressure Vessels etc., fabricated during or before
World War-II, cracked in cold weather / temperature . This lead to brittle
Definition of Cryogenics: In Romans, Cryo means super cold and genics means knowledge or science. In Greek,
Crogenics is to attain low temperature.
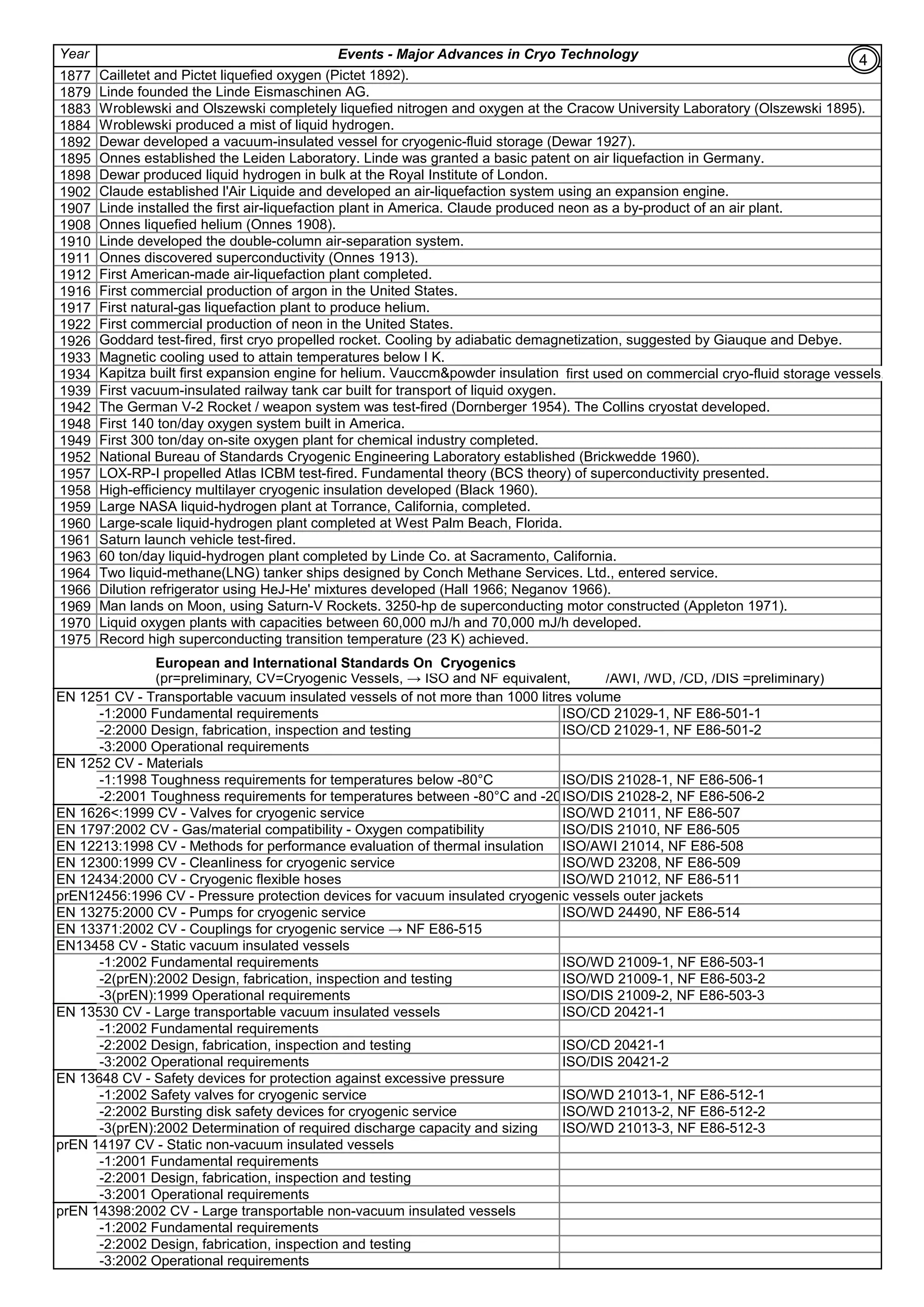
Achieving of Cryogenic Temperatures: The process is like normal refrigeration cycle to make ice . Instead of
Freon/Refrigerant, Cryogens (Liquid Helium, liquid Nitrogen, petroleum gases etc) or mixture of them(MRC) are used.
(6). Superconductivity: Superconducting electromagnets are used in the Particle Accelerators. The facilities require very
powerful magnetic flux that conventional electromagnets could be melted by the electric currents they carry. Liquid helium
cools the cable through which the currents flow to about 4 K , allowing much stronger currents to flow without generating
heat by electrical resistance. The electrical resistance at sub-zero temperature is near zero.
(A). Most BCC Metals, Iron, CS & low alloy steels, Moly, Nb, Zinc, elastomeric &
plastics materials: they generally, on cooling from room temp. to cryo temperature
(
‐
- 273ºC), the following happens: (1). Tensile & Yield Strength increases. , (2). %
Elangation(ductility) decreases, (3). Youngs Modulus increases, (means little
deflection/rigid), (4). Coefficient of thermal expansion continue to decrease and it is
negligible, near to absolute zero. (5). impact Energy decreases and the structure
becomes brittle.
(B). Most FCC Metals, Cu, Ni, Cu
‐
Ni alloys, Al & its alloys, Austenitic Stainless Steels
(with min 7%Ni), Zirconium, Titanium, Teflon, polyurethane, pyrex glass, 9% Ni steel
are generally safe for cryo temp. range.
[1]. Effects of Low Temp & Cryo temp on Toughness and Ductility:
(Unit Joules, 1J = 0.738 ft.lb)
(8). As a cooling medium: Liquid N2 & Liquid He are used for cooling purpose on infrared cameras, thermal imagers,
high vacuum pumps, storing human blood, tissues, cells etc. For testing at low temperature / cryo temperature : weld test
coupons/PTC, impact test specimens, samples, valves, equipments etc.
(E). 20J (15 ft.lb) impact energy at Operating Temperature is considered minimum to control brittle failure, at low temp /
Cryogenic Engineering Applications. Allowances are added for further safety.
[2]. Effect of Cryo temp on Electrical Resistance of Material :
The Electrical Resistance, reduces near to zero Ohms(Ω), as the temp. is lowered , near to absolute zero. Zero Electrical
Resistance at Zero absolute temperature, is called Super Conductivity.
(2). LNG Transport: Cryogenic gas liquefaction techniques is the storage and transportation of liquefied natural gas
(LNG), a mixture largely composed of methane, ethane, and other combustible gases. Natural gas(Methane, CH4) is
liquefied at
‐
-163ºC, causing it to contract to 1/600th of its volume at room temperature and making it sufficiently compact
for swift transport in specially insulated tankers to a far away distant places(say from Gulf Countries to Japan, Korea).
(1). Liquefaction & Gas Separation: Using cryo technology, Air is separated into Oxygen, nitrogen and other
constituents. Cryotechnology is used to separte petroleum products like Methane, Ethane etc. Oxygen(liquid & gas) is used
in Metallaurgical units, Hospitals, Space Applications etc.
(3). Food Preservation: Very low temperatures are also used for preserving food, simply and inexpensively. Produce is
placed in a sealed tank and sprayed with liquid nitrogen. The nitrogen immediately vaporizes, absorbing the heat content of
the produce.
(4). Cryo
‐
medicine & Cryosurgery: A low
‐
temperature scalpel or probe can be used to freeze to cut & kill unhealthy
tissue & unwanted human growth(cancer or dead body part). The resulting dead cells are removed through normal bodily
processes. The advantage : freezing the tissue rather than normal cutting, it produces less bleeding
(5). Space Application: Cryo fuel/Propellants (Liquid Hydrogen & Oxygen) were first used in Rockets, like Apollo-11
(Saturn-V) which took man to moon in 1969 and first used in Space-Shuttle “Columbia” in 1981
(7). Brittle Nature: Many materials at cryo temp range, have brittle structure (%Elangation <5%, is considered brittle
fracture). Food grains, elastic materials like rubber, sticky material, plastics and tools used to metal cutting etc are sub
‐
cooled to cryo temp range and machined and also ground to powder, as the material is brittle and easy to machine. Dead
Human body, at cryo temp, is shaved, at 1 mm layers and photographed to make 3D model for training/analysis/treatment
by Doctors. Some steels are treated at
‐
- 185ºC. Tools: Life of metal tools increases to between 200
‐
400% of the original
life expectancy using cryo tempering instead of heat treating.
1](https://image.slidesharecdn.com/cryogenicsinbrief-2-200919083642/75/Cryogenics-in-Brief-1-2048.jpg)