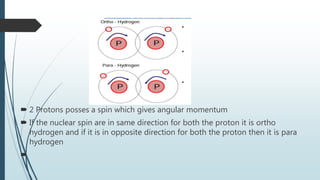
This document discusses cryogenic technology and applications of cryogenic fluids. It introduces cryogenics as the science of low temperatures below 123K and lists some common cryogenic fluids like hydrogen, helium, oxygen and nitrogen. It then describes the liquefaction processes for gases using simple systems like Linde-Hampson and more advanced ones like Claude and Collins systems which use expansion engines to further lower temperatures. Finally, it outlines several applications of cryogenics in areas like superconductivity, medicine, space and cryonics.