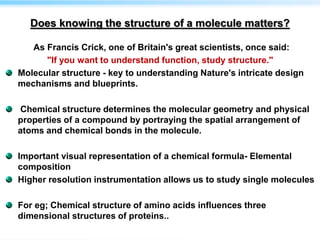
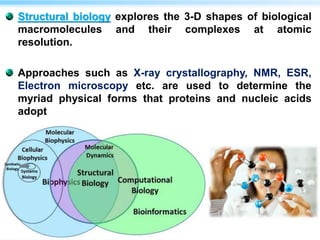
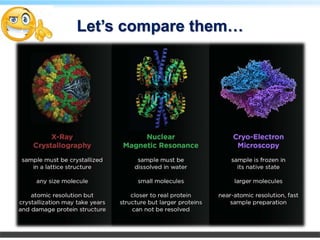
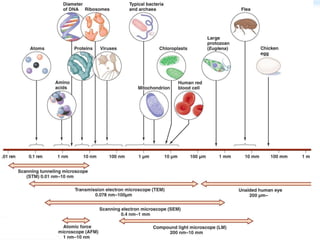
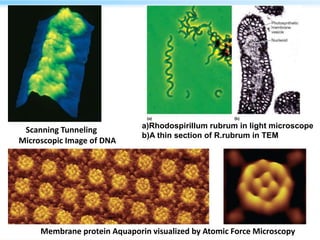
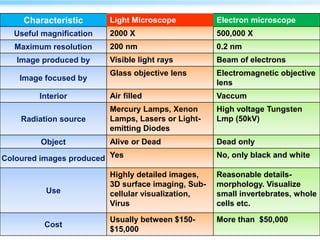
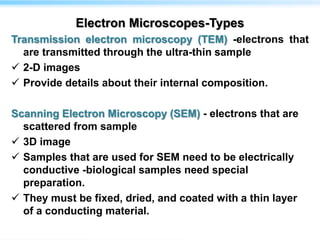
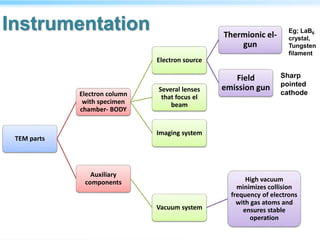
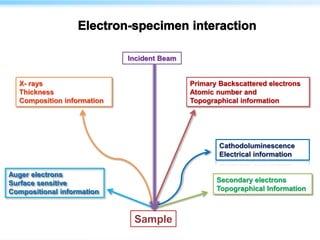
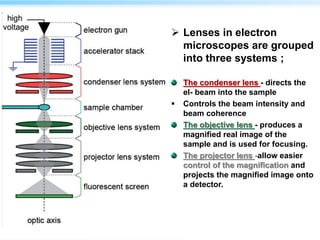
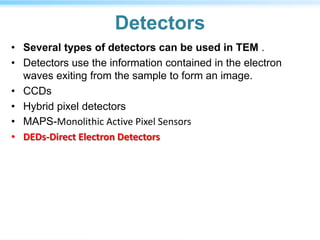
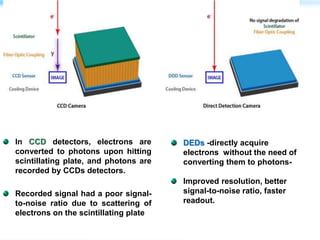
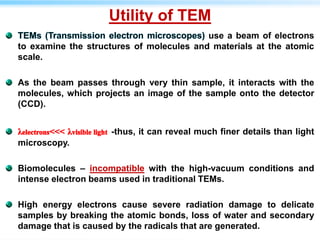
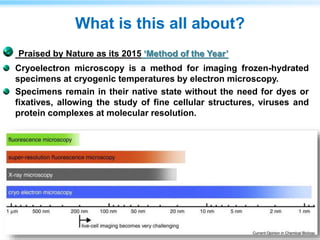
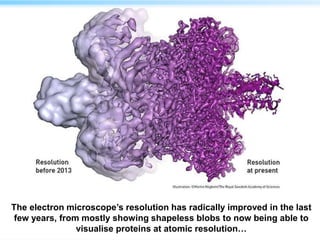
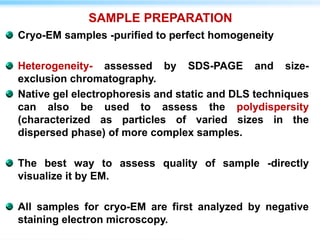
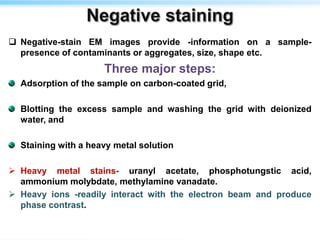
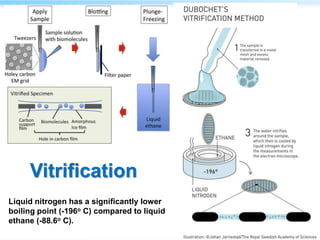
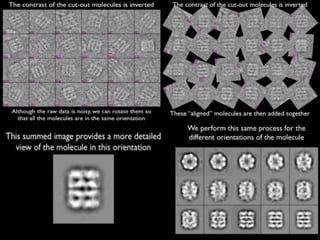
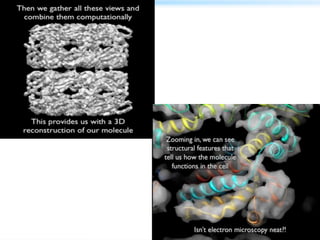
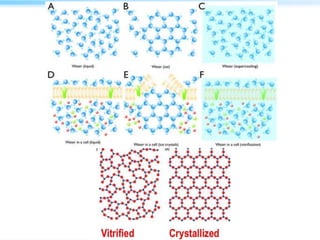
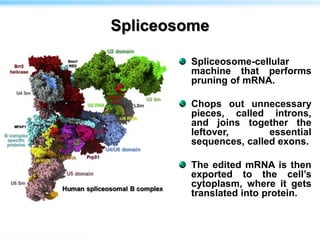
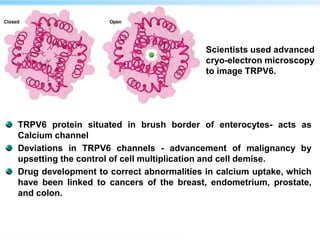
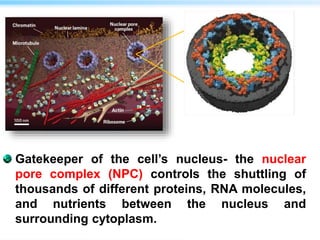
This document discusses cryo-electron microscopy (cryo-EM), a technique used to image biological macromolecules in their native frozen state without staining or chemical fixation. It describes how cryo-EM overcomes limitations of traditional transmission electron microscopy by rapidly freezing samples to vitrify water and prevent ice crystal formation. The document outlines key developments in cryo-EM including contributions from Richard Henderson, Jacques Dubochet, and Joachim Frank who were awarded the 2017 Nobel Prize in Chemistry. Examples of structures solved using cryo-EM such as the Zika virus and nuclear pore complex are provided. Advantages and applications of cryo-EM are summarized.