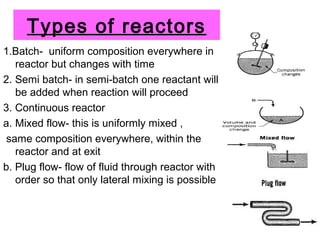
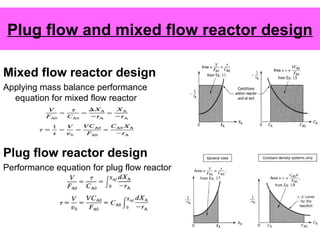
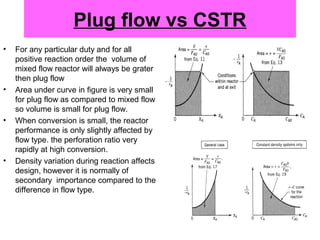
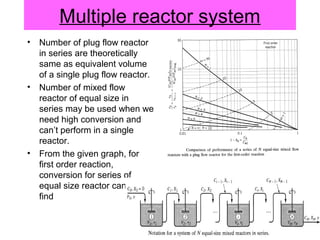
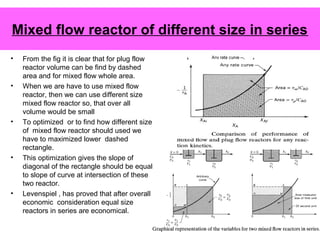
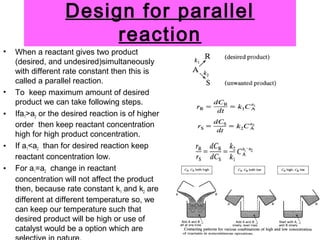
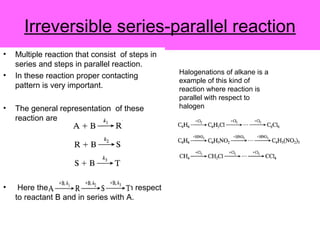
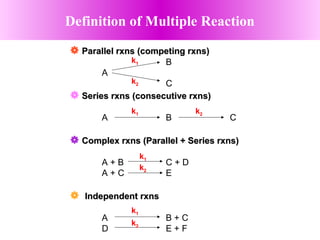
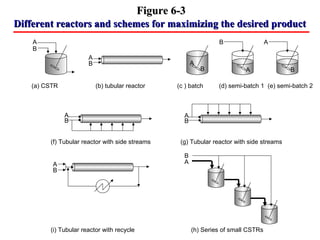
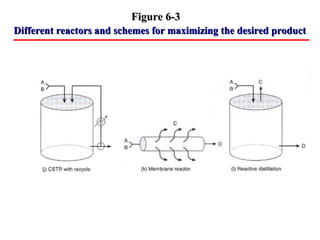
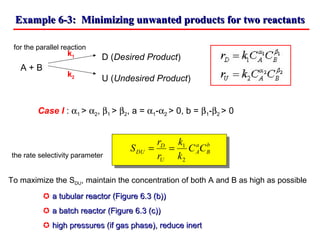
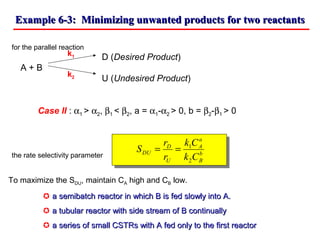
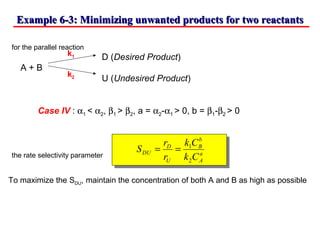
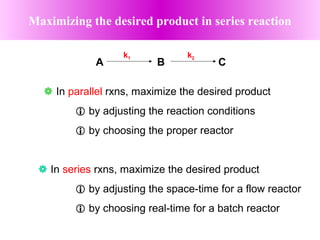
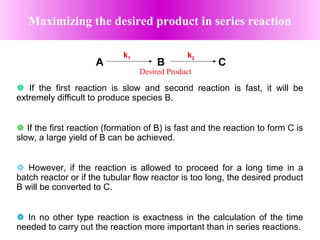
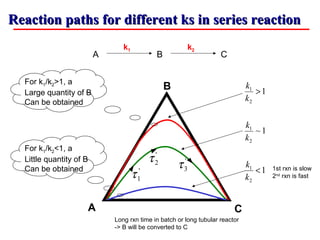
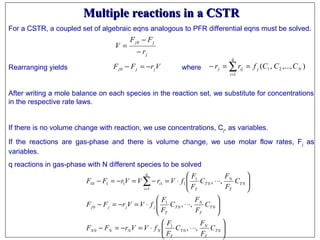
This document discusses reactor design for multiple reactions. It begins by describing types of reactors including batch, semi-batch, and continuous. Design parameters like volume, flow rate, concentrations, kinetics, temperature, and pressure are discussed for reactor selection. Equations for mixed flow and plug flow reactor design are presented. Plug flow reactors are generally smaller than continuous stirred tank reactors (CSTRs) for a given conversion. Methods for maximizing the desired product in parallel and series reactions include adjusting conditions like concentrations, temperatures, and choosing the proper reactor type. Multiple reactor systems with reactors in series or mixed flow reactors of different sizes can be used for high conversions that a single reactor cannot achieve.