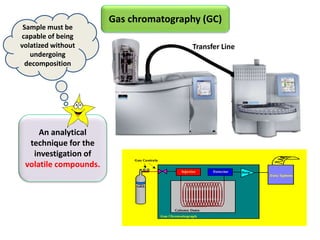
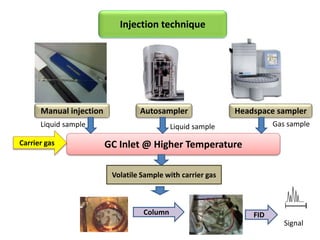
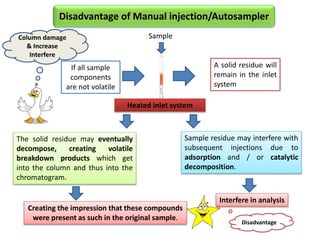
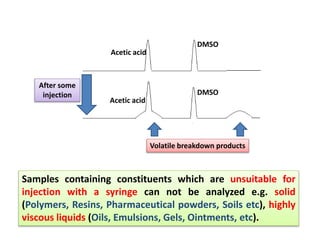
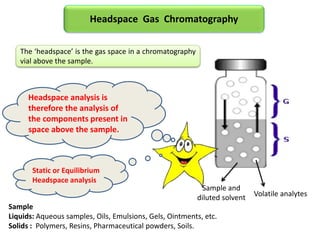
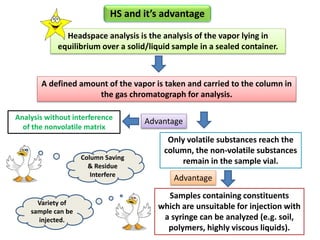
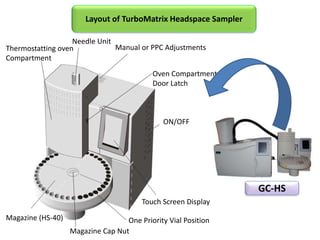
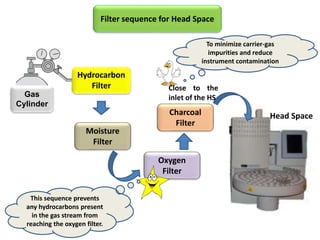
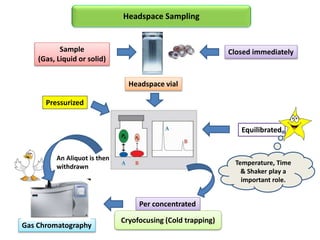
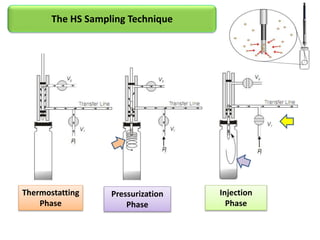
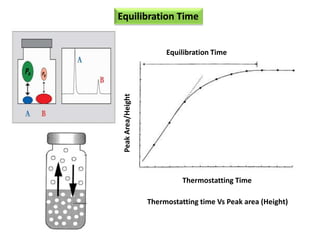
1) Headspace gas chromatography allows the analysis of volatile compounds in samples without directly injecting the sample into the GC. This prevents non-volatile residues from interfering.
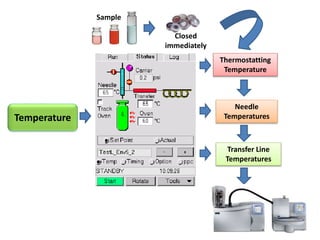
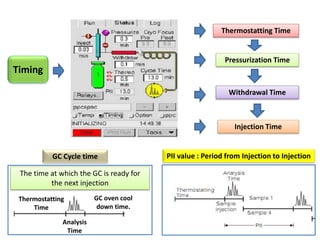
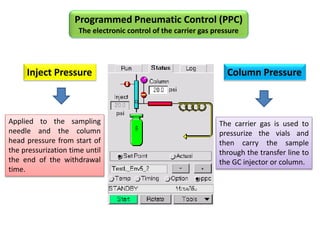
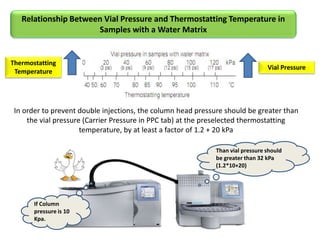
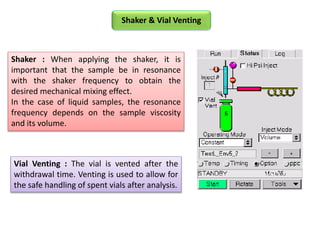
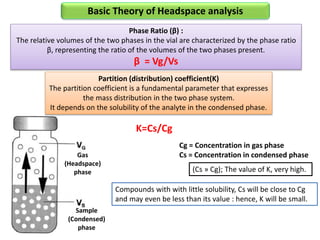
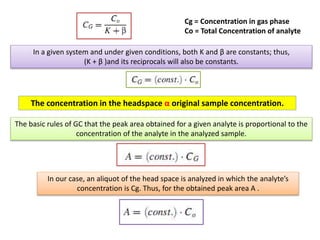
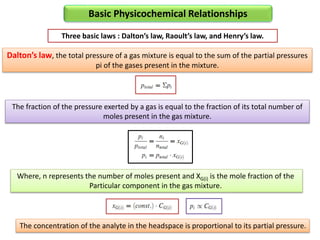
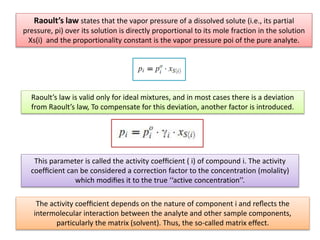
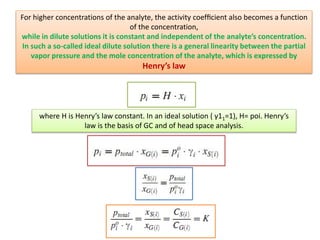
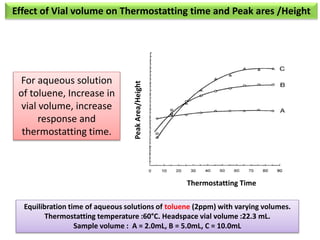
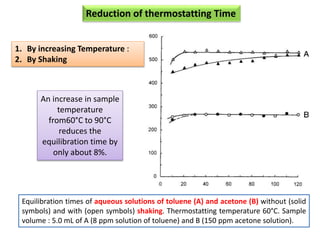
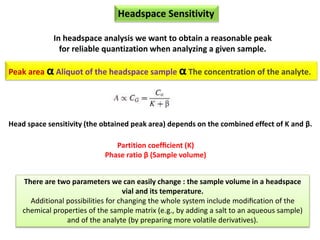
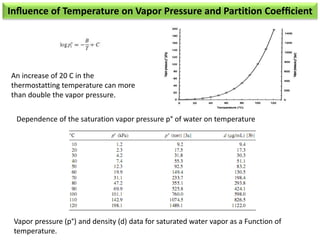
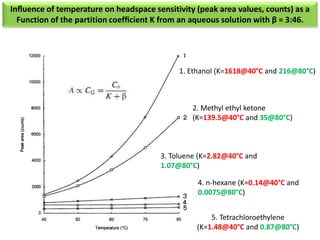
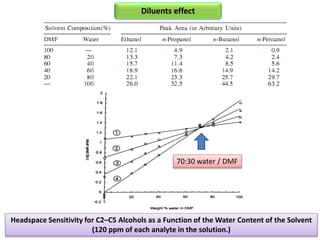
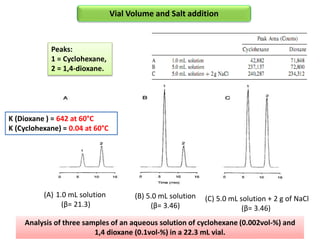
2) Key factors that affect headspace sensitivity and analysis include temperature, phase ratio (sample volume), partition coefficient, and concentration - which influence the concentration of analyte in the headspace.
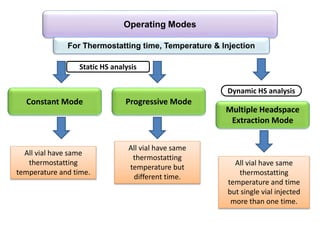
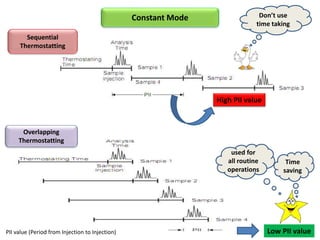
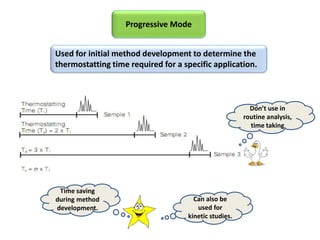
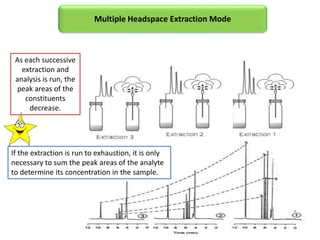
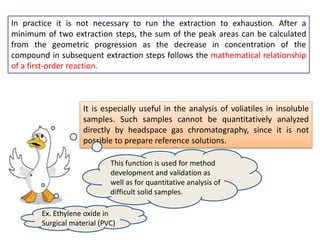
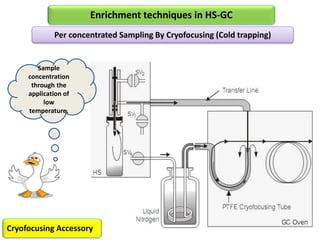
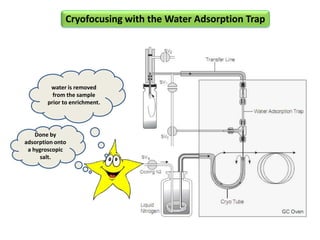
3) Techniques like multiple headspace extraction and cryofocusing can be used to concentrate analytes and improve detection of compounds in complex samples.