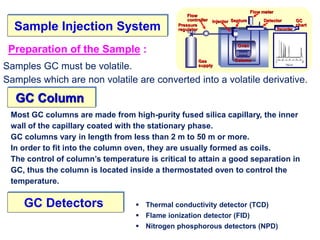
Gas chromatography is a technique used to separate volatile organic compounds based on differences in their partitioning behavior between a mobile gas phase and a stationary phase in a column. The sample is injected and carried by an inert mobile gas through the column containing a coated stationary phase. Components are separated as they interact differently with the stationary phase and emerge from the column at different retention times, allowing for qualitative and quantitative analysis. There are two main types: gas-solid chromatography uses a solid stationary phase while gas-liquid chromatography is most widely used with a liquid phase.