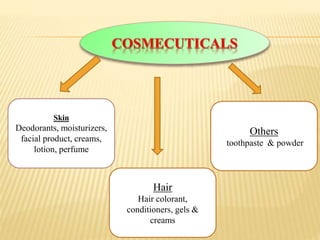
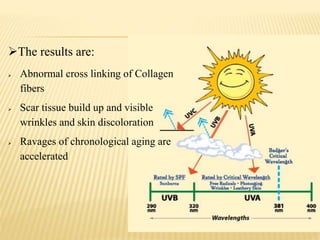
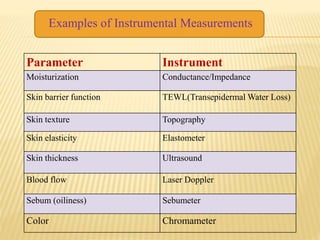
This document defines cosmeceuticals as ingredients with medicinal properties that provide topical benefits and protect against skin damage. Cosmeceuticals were coined in 1980 and are not officially recognized by the FDA, which classifies products as drugs or cosmetics. Common cosmeceutical ingredients include antioxidants, peptides, retinoids, and sunscreens which treat signs of aging and damage from UV rays. Their benefits and safety testing were also discussed.