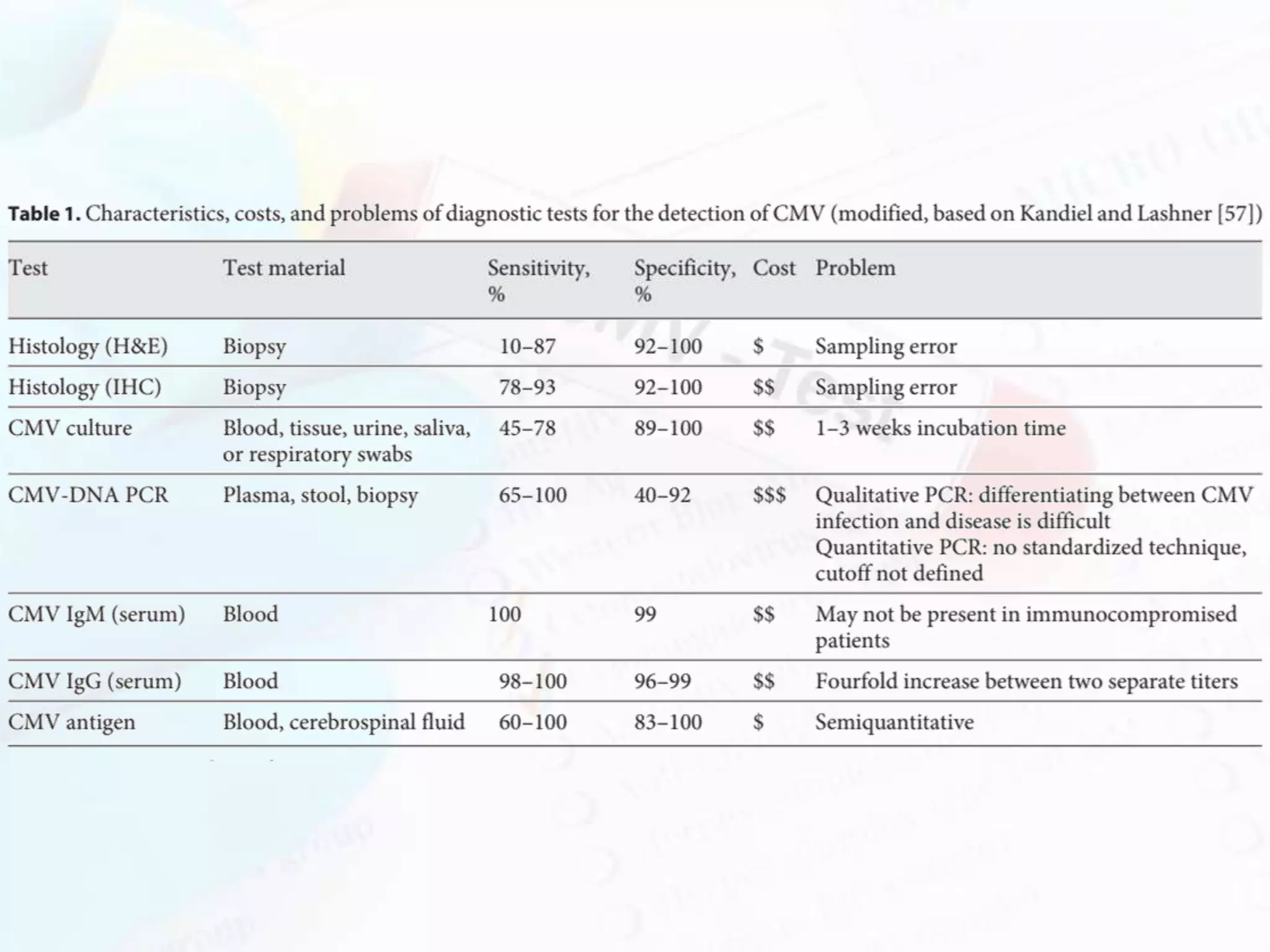
Congenital cytomegalovirus (CMV) infection is a significant global health issue, being a leading cause of non-hereditary sensorineural hearing loss and other neurodevelopmental disabilities in newborns. Transmission occurs both horizontally and vertically, with varied clinical manifestations depending on the infant's immune status, ranging from mild conditions to severe disease. Diagnosis relies on laboratory tests, and management may include antiviral therapies, monitoring for complications, and long-term follow-up due to potential late sequelae.









































![• Viral sepsis-like
syndrome
• Pneumonitis
• Myocarditis
• Severe hepatitis
• Enterocolitis
• Severe and refractory
thrombocytopenia
• Sight-threatening retinitis
• Severe neurologic disease
• Underlying primary
immune disorder (e.g.,
severe combined
immunodeficiency [SCID])
regardless of degree of
symptoms
Indication:
Life-threatening disease —with any of the following:](https://image.slidesharecdn.com/congenital-cytomegalovirus-infection-191215171934/75/Congenital-cytomegalovirus-infection-44-2048.jpg)



















![Late sequelae of symptomatic congenital CMV included:
• Hearing loss
• Intellectual disability [IQ <70]
• Microcephaly Strabismus
• Dental disease
• Seizures
• Cortical visual impairment
• Chorioretinitis
• Cerebral palsy
• Death after the newborn period](https://image.slidesharecdn.com/congenital-cytomegalovirus-infection-191215171934/75/Congenital-cytomegalovirus-infection-64-2048.jpg)













