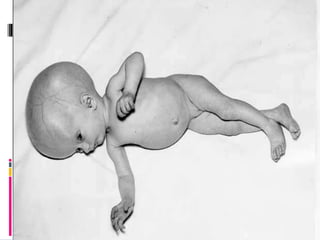
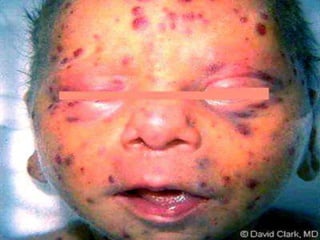
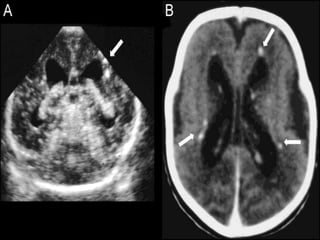
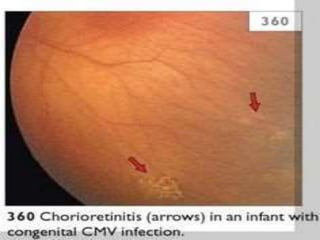
The TORCH complex is a set of perinatal infections that can be transmitted from mother to fetus, including toxoplasmosis, rubella, CMV, and herpes. These infections pose risks for severe fetal anomalies and can cause fetal death. Toxoplasmosis is caused by a parasite and transmitted through undercooked meat, soil, or water. Rubella virus causes congenital defects especially if the mother is infected in the first trimester. CMV and herpes viruses are also transplacentally transmitted and can cause issues like hearing loss, jaundice, or brain damage in the fetus or newborn. Diagnosis involves tests on maternal, amniotic, and neonatal samples. Treatment focuses on supportive