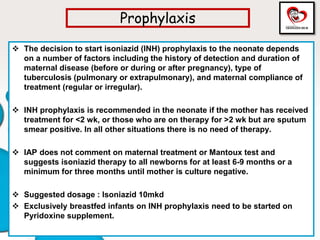
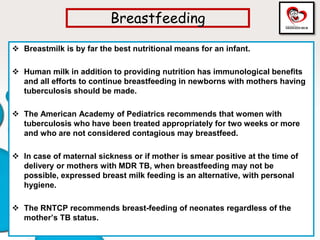
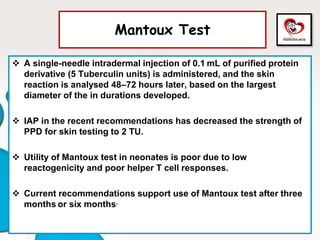
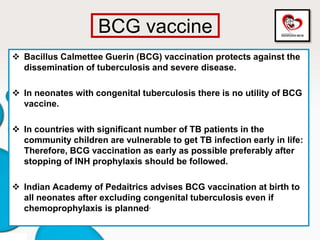
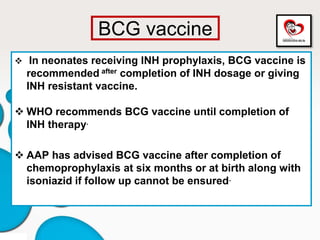
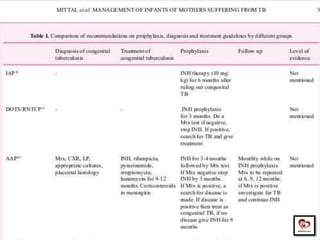
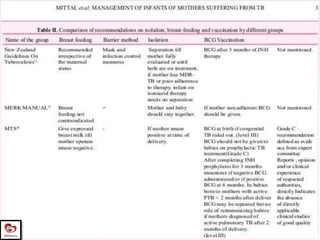
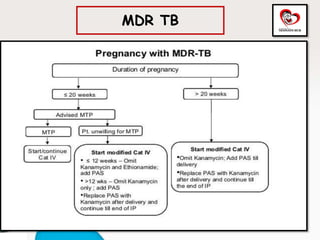
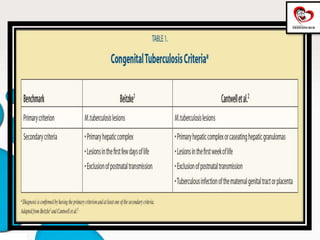
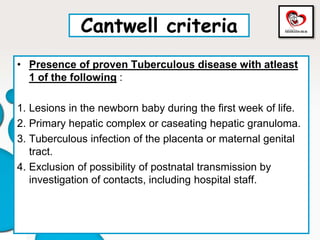
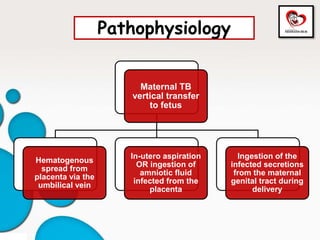
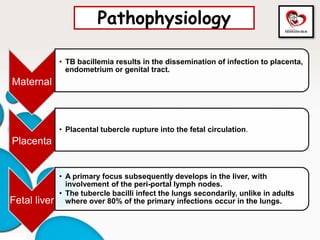
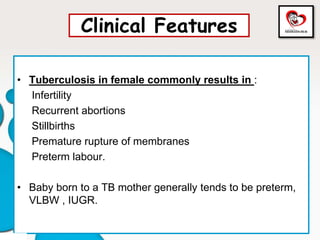
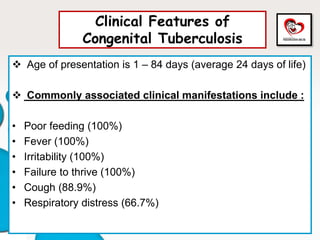
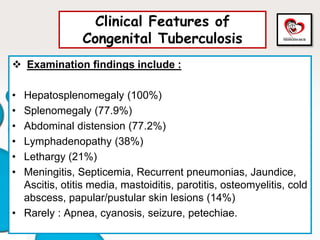
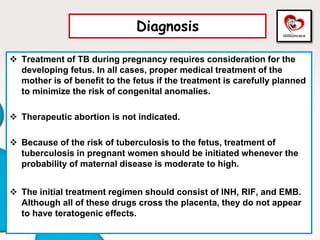
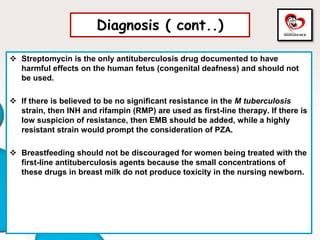
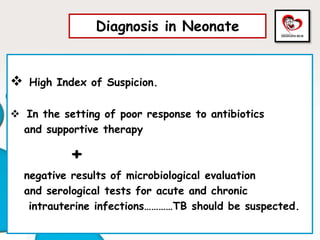
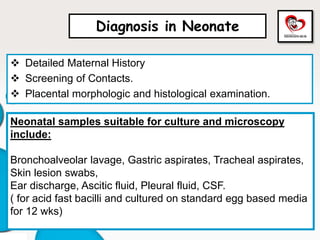
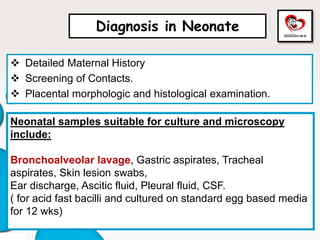
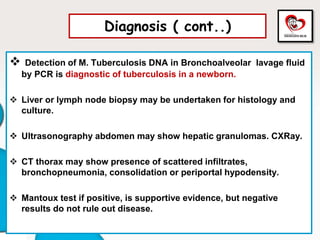
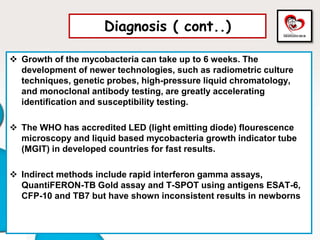
Congenital tuberculosis is a serious form of TB in neonates, with high morbidity and mortality, and is diagnosed based on specific clinical criteria and maternal history. Treatment involves careful management of the mother and neonate, using first-line antituberculosis medications while considering the fetus's safety. Breastfeeding is encouraged with necessary precautions, and vaccination strategies, including BCG, are recommended based on maternal and neonatal circumstances.





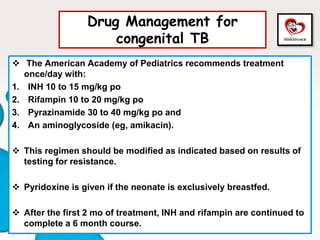
![Drug Management for
congenital TB (cont..)
When the CNS is involved, initial therapy also includes
corticosteroids (prednisone 2 mg/kg po once/day [maximum 60
mg/day] for 4 to 6 wk, then gradually tapered).
Other therapy continues until all signs of meningitis have
disappeared and cultures are negative on 2 successive lumbar
punctures at least 1 wk apart.
Therapy can then be continued with INH and rifampin once/day or
twice/wk for another 10 mo.
Corticosteroids may also be considered for infants and children
with severe miliary disease, pleural or pericardial effusions, or
endobronchial disease or those with abdominal TB.](https://image.slidesharecdn.com/congenitaltb-200812104925/85/Congenital-Tuberculosis-Updated-in-2020-23-320.jpg)