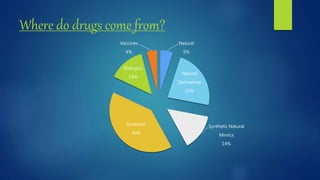
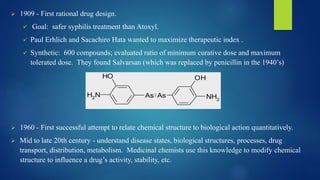
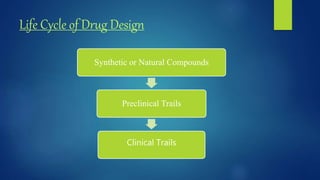
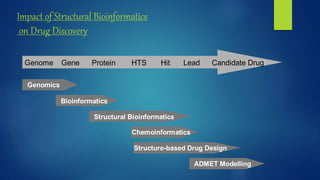
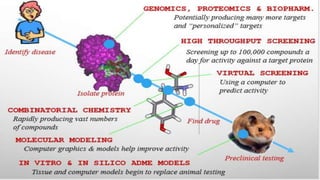
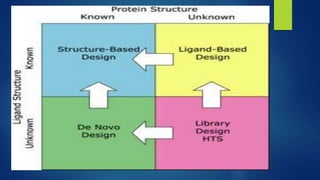
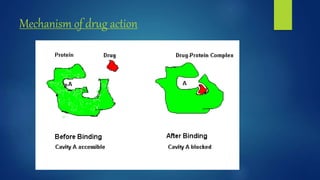
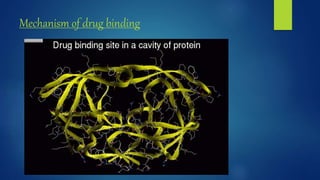
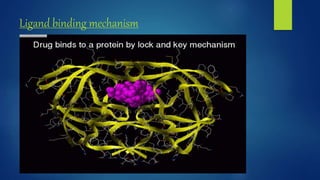
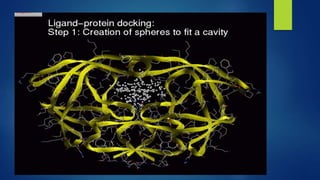
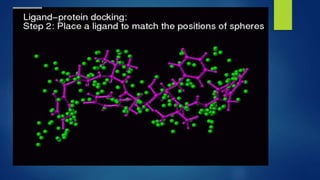
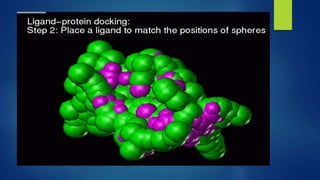
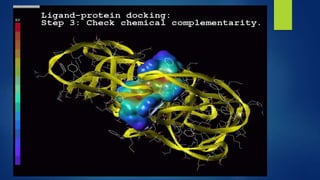
Computer aided drug design uses computational approaches to aid in the drug discovery process. There are several key approaches including ligand based approaches which identify characteristics of known active ligands, target based approaches which use information about the biological target, and structure based drug design which utilizes 3D structural information. The main steps in drug design include target identification and validation, lead identification and optimization, and preclinical and clinical trials. Computational tools are used throughout the process for tasks like molecular docking, ADMET prediction, and structure activity relationship analysis.