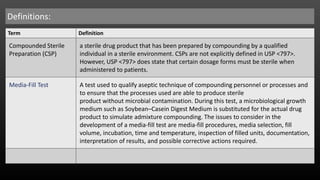
The document outlines guidelines and responsibilities for personnel involved in compounding sterile preparations (CSPs), emphasizing the importance of training and compliance with aseptic techniques. It details the media-fill test procedures for low, medium, and high-risk level CSPs, which assess the ability of compounding personnel to maintain sterility and adhere to required safety standards. Additionally, it specifies the necessary qualifications for compounding personnel and the evaluation processes to ensure the integrity and safety of compounded products.








![Media-Fill Challenge Testing
Media-fill testing is used to assess the quality of the aseptic skill of
compounding personnel.
Media-fill tests represent the most challenging or stressful
conditions actually encountered by the personnel being evaluated
when they prepare particular risk level CSPs and when sterilizing
high-risk level CSPs. Media-fill challenge tests that simulate high-risk
level compounding are also used to verify the capability of the
compounding environment and process to produce a sterile
preparation.
Commercially available sterile fluid culture media, such as Soybean–
Casein Digest Medium (a.k.a - trypticase soy broth or trypticase
soy agar [TSA] )shall be able to promote exponential colonization
of bacteria that are most likely to be transmitted to CSPs from the
compounding personnel and environment.
Media-filled vials are generally incubated at 20° to 25° or at 30° to
35° for a minimum of 14 days.
If two temperatures are used for incubation of media-filled samples,
then these filled containers should be incubated for at least 7 days
at each temperature. Failure is indicated by visible turbidity in the
medium on or before 14 days.](https://image.slidesharecdn.com/compoundingpersonnel-150318135725-conversion-gate01/85/Compounding-personnel-10-320.jpg)












