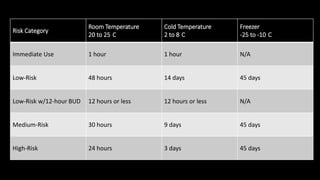
The document explains the concept of beyond-use dating (BUD) for compounded sterile products (CSPs), emphasizing that BUD is distinct from the expiration date and is based on factors like chemical stability and sterility limits. It outlines the guidelines for determining BUDs according to the United States Pharmacopeia (USP) and highlights that BUDs must be shorter than manufacturers' expiration dates. The document also notes that while USP guidelines can be exceeded, this requires independent sterility testing.



















![USP <797> provides recommended
BUD guidelines based on CSP
microbial contamination risk category
For more information on CSP risk category……[link to CSP Risk Category presentation]](https://image.slidesharecdn.com/understandingbeyond-usedatingforsterilecompounds-150318142224-conversion-gate01/85/Understanding-Beyond-Use-Dating-for-Sterile-Compounds-20-320.jpg)