Embed presentation
Download to read offline
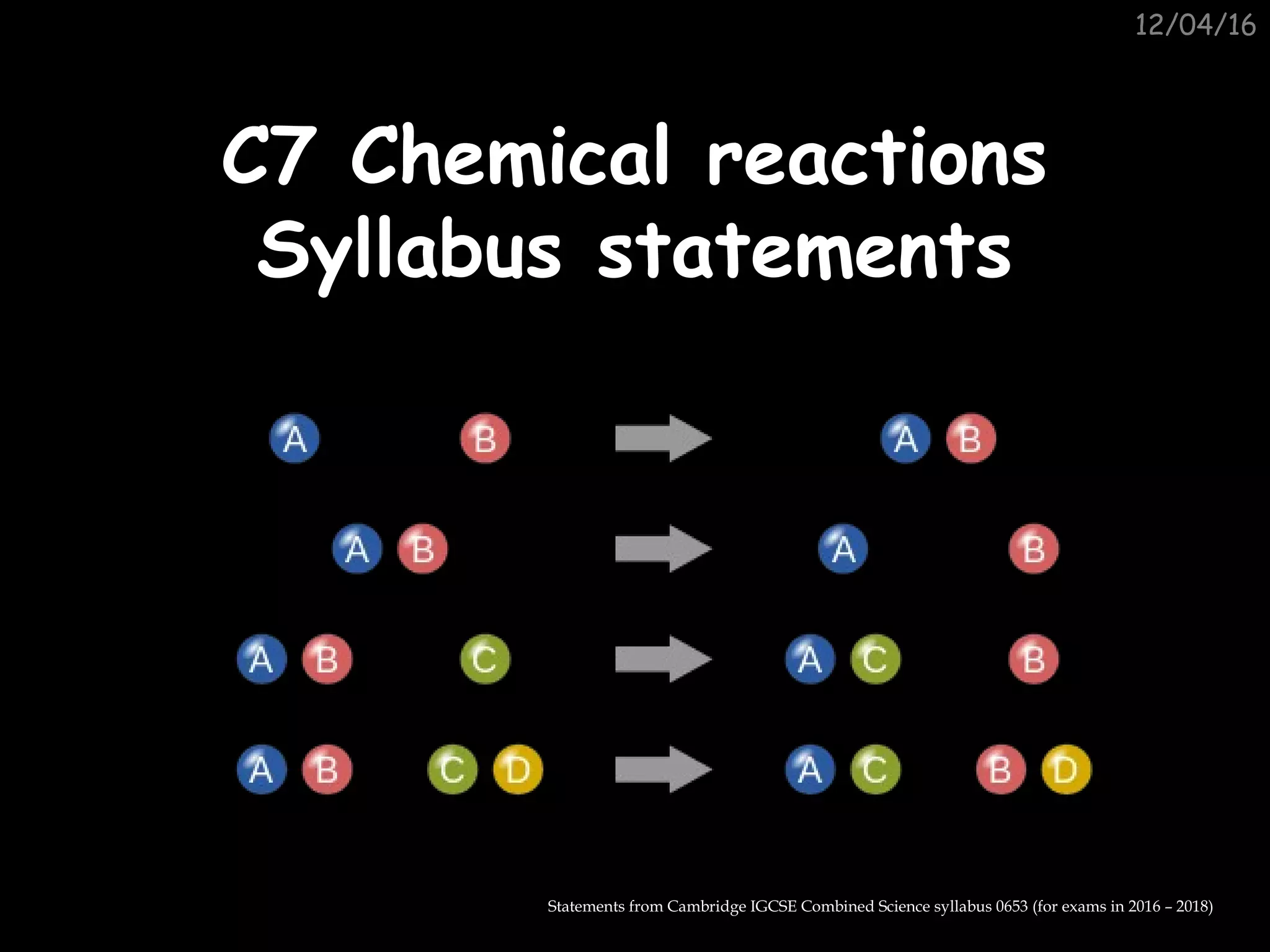



The document contains statements from the Cambridge IGCSE Combined Science syllabus 0653 covering chemical reactions and rates of reaction. It states that students should be able to describe how concentration, particle size, catalysis and temperature affect the rate of reaction and explain the effects of temperature and concentration in terms of particle collisions. It also says students should be able to describe methods to investigate gas-evolving reactions, interpret rate data, and define catalysts as substances that increase reaction rates but remain unchanged.



