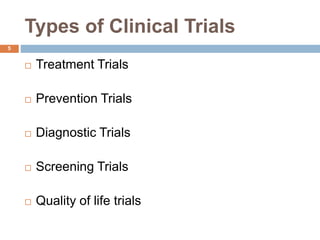
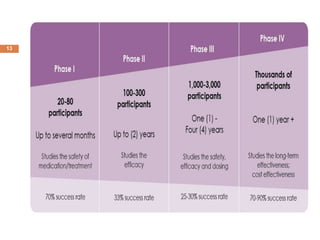
This document outlines the phases of clinical trials, from Phase 0 to Phase IV. It defines clinical trials as prospective biomedical studies on human subjects to answer questions about interventions. The objectives are listed as diagnosing or treating disease, preventing disease or early death, or changing behaviors. The phases are described in order from exploratory studies in small groups in Phase 0 to post-marketing surveillance trials in large populations in Phase IV. The importance of clinical trials is that they follow strict scientific standards to protect patients and produce reliable results, as a final stage of research after laboratory and animal testing.