This document provides an overview of clinical trials, including:
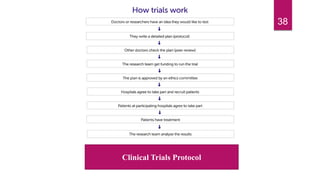
- Definitions of clinical trials and their importance in testing medical treatments.
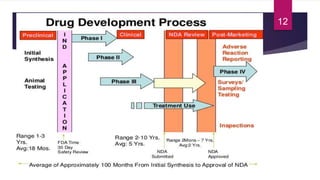
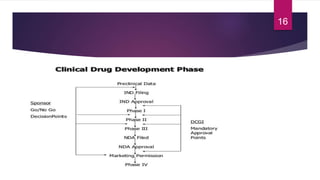
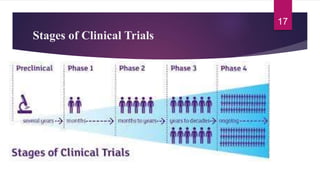
- The various phases of clinical trials (Phases 0-IV) and their objectives in evaluating safety, efficacy, and effectiveness.
- The roles of institutional review boards, peer review, and regulatory approval in the clinical trial process and new drug application.