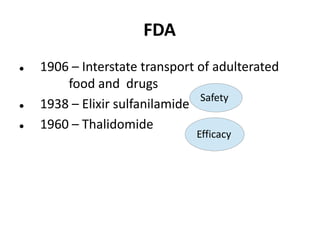
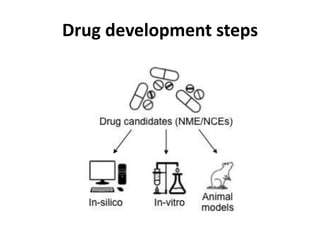
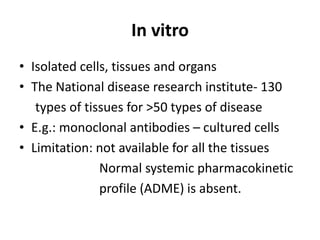
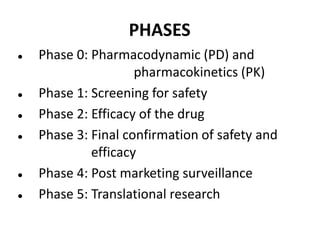
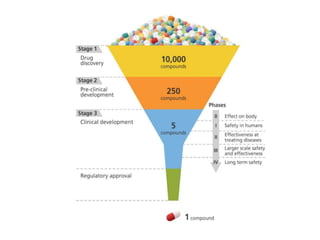
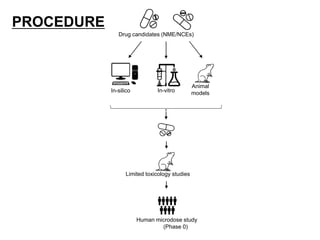
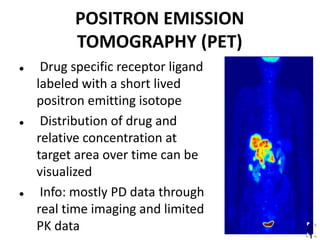
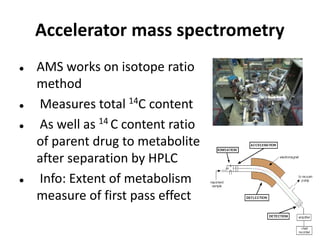
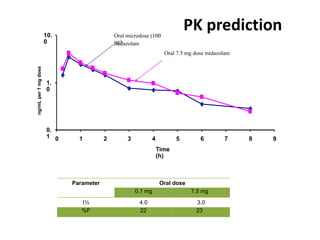
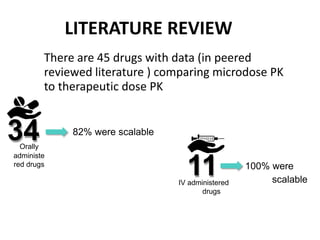
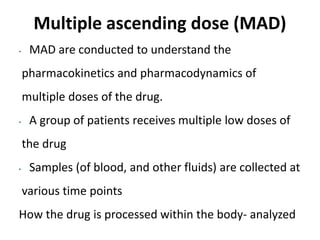
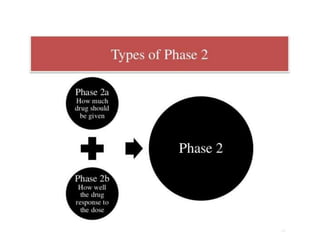
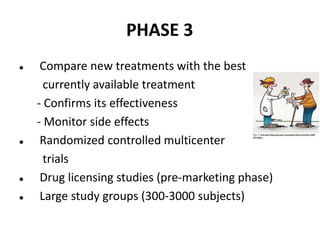
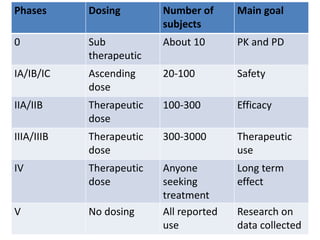
Clinical trials involve systematic studies of new drugs or therapies in human subjects to generate safety and efficacy data. They generally proceed through several phases:
- Pre-clinical trials use in silico, in vitro, and animal studies to obtain early pharmacological and toxicity data before human testing.
- Phase 1 trials involve small groups of subjects and focus on safety, tolerability, and identifying safe dosage levels.
- Phase 2 trials administer the drug to larger groups of patients with the target disease to further evaluate safety and assess efficacy.
- Phase 3 trials involve large patient populations to confirm efficacy and further monitor safety and side effects.
- Phase 4 trials collect longer term safety and efficacy data after market approval and identify