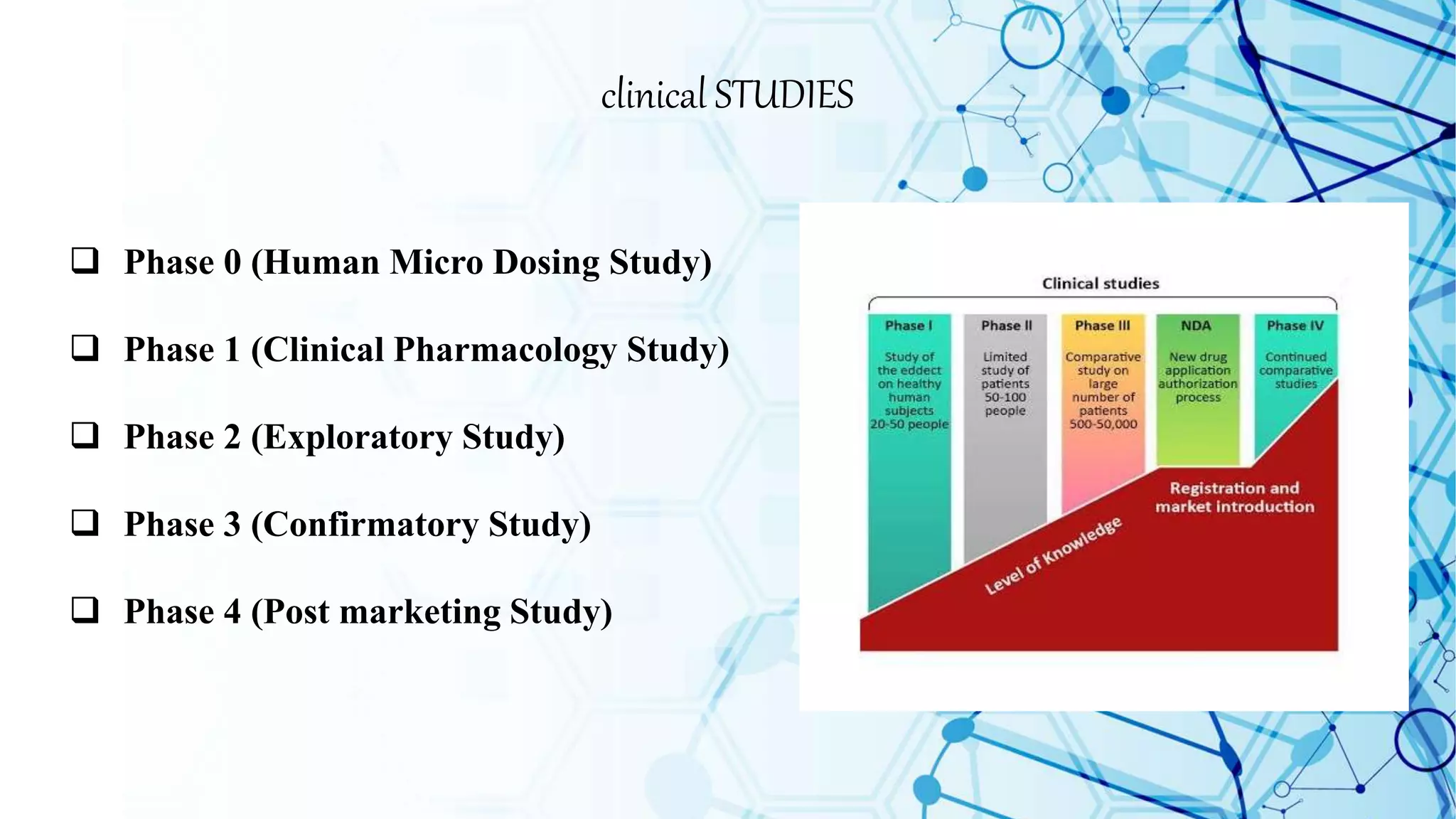
Clinical trials involve testing new drugs and medical treatments on humans to determine safety and efficacy. The process involves several phases:
Preclinical studies first test the treatment on animals. If successful, phase 1 studies involve small human trials to evaluate safety. Phase 2 studies expand to hundreds of people to determine effective dosages. Phase 3 trials involve thousands of people to further confirm safety and effectiveness compared to existing treatments. If successful, the results are submitted to regulatory agencies for approval, and phase 4 post-marketing studies monitor long-term safety and efficacy in large patient populations. Together, the clinical trial phases provide critical data on medical treatments before they are approved and widely available to patients.