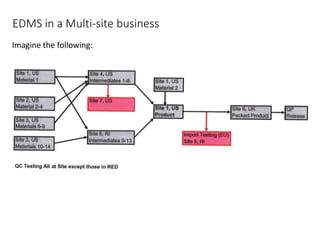
The document outlines the critical roles of Electronic Data Management Systems (EDMS) and Document Management Systems (DMS) in multi-site businesses, emphasizing the need for control, access, and traceability of documents and data. It distinguishes between various systems such as LIMS, deviations, and MRP, describing their specific requirements and functionalities. The importance of compliance with 21 CFR Part 11 and adherence to GAMP guidelines is also highlighted to ensure security, audit trails, and suitability for regulatory standards.